Cancer screening, part I
Policy recommendations on governance, organization and evaluation of cancer screening
This is a part I of chapter Cancer screening (Cancon work package 9) of the Guide. See part II of the chapter.
See the full Guide and other chapters as pdf's.
Cancon Guide is the main delivery of the joint action.
Contents of Cancer screening part I:
Main messages
Introduction
Methods
Governance of cancer screening
Organizational requirements
Integrated evaluation, part I
Cancer screening part II:
Integrated evaluation, part II
Potential new cancer screening programmes
Summary and conclusions
References
Main messages
- National structures for governance of screening are here identified as important requirements for evidence-based decision-making and for establishing adequate legal, financial and organizational frameworks for effective cancer screening programmes with integrated quality assurance. We recommend transparent, structured and publicly documented decisionmaking, informed political commitment and broad stakeholder involvement in order to build strong professional support for the aims and means of the screening programme. Governance structures recommended here are currently lacking in many European settings, which may contribute substantially to inequalities in cancer prevention outcomes observed both between and within countries.
- Organization for the practical implementation and the continual gradual improvement of population-based cancer screening programmes further requires careful coordination of this multistep process with feedback and corrective modification at each step, plus revolution of the quality circle. Information systems that permit registration and monitoring of process and outcome are crucial for maintaining current levels of quality, and for guiding further improvement.
- Evaluations of the benefit-harm balance and cost-effectiveness of screening are required periodically for existing programmes and prospectively for new screening programmes. The population targeted by screening have an ethically mandated right to clear information on benefits and harms for an informed choice about participation. Indicators for equity in participation and health outcomes need to be included in the routine quality assurance capabilities of population-based screening programmes.
- New screening programmes require step-wise decision-making which includes the establishment of evidence of effectiveness, benefits that outweigh the harms and costeffectiveness. Once evidence exists to support these criteria, implementation research in each country is needed to assess the feasibility of fulfilling the national requirements in practice. In light of currently available evidence, some prostate cancer screening policies may be costeffective but questions remain on the optimal benefit-harm balance. Forthcoming results of European trials are expected to inform policy-making on lung cancer screening in Europe. New trials need to be financed to investigate optimal strategies for gastric cancer screening.
Introduction
Screening refers to the use of relatively simple tests across an apparently healthy population in order to identify individuals who have risk factors or an unrecognized disease or defect. Box 4.1 outlines the terms used within this chapter when discussing aspects that impinge on screening.
A screening test is not intended to be diagnostic, and persons with a positive or suspicious finding must be referred for a confirming diagnosis and necessary treatment (1). It is essential that screening identifies those who are more likely to be helped than harmed by further tests or treatment to reduce the risk of a disease or its complications (2). The WHO criteria for screening (1) date from 1968 and have since been refined to highlight the importance of evidence of an acceptable balance between benefit and harm, integrated monitoring and evaluation, equity, and informed choices based on available evidence (Box 4.2) (3). Based on the criteria by WHO and others (1,3,4), three conditions determine the relevance of a screening programme: there has to be evidence for the effectiveness of screening, that the benefits of screening outweigh the harms and that screening is cost-effective (4). These refined criteria are relevant for decision-making concerning screening programmes in the 21st century and form a backdrop for discussion of the collection of evidence before implementation and in routine monitoring and the decision-making processes concerning screening programmes in this chapter.
Box 4.1 Terms used in Chapter 4
Audit
Audit is the systematic examination of current practice against guidelines or a defined desired standard. Cancer screening audits examine the screening history of cancer patients and controls in order to identify and quantify failures of the screening process and the potential for improvement.
Governance
Governance in the health sector refers to a wide range of steering and rule-making functions carried out by governments and other decision-makers to achieve and develop the national health policy objectives.
Opportunistic testing
Opportunistic testing is initiated by individual members of the public or their health advisors. It may or may not be based on national guidelines on intervals, target population and screening tests.
Population-based screening
Population-based screening is conducted according to nationally implemented guidelines defining who should be invited, how frequently they should be screened and how any abnormalities detected should be followed up and treated. The screening programme identifies each individual to be personally invited from a population register. Adherence to national guidelines is monitored in a screening register. Population-based screening programmes generally require a high degree of organization in order to assure that the invitational activities are performed reliably and effectively and are adequately coordinated with the subsequent steps in the screening process.
Risk-stratified screening
In risk-stratified screening, the specific screening policy regarding screening ages, intervals, tests and follow-up algorithms is based on the risk profile of a group of individuals in the population. This may include no screening for those at lowest risk and an unfavourable expected benefitharm ratio. Risk-stratified screening should not be confused with clinically initiated risk profiling, for example genetic testing of patients with breast cancer and their relatives for follow-up of BRCA positive status. Risk-stratified approaches have a theoretical potential to improve overall cost-effectiveness and benefit-harm ratios of population-based screening programmes.
Stewardship
Stewardship in health implies that the ministries in charge of health assume the ultimate responsibility for the management of the national resources to the health benefit of their entire population, by directing the establishment of as good and fair health system as possible and by promoting health aspects in all policies.
Quality assurance
Quality assurance encompasses activities intended to assure and improve quality at all levels of the screening process in order to maximize benefits and cost-effectiveness while minimizing harms. The concept includes the assessment or evaluation of quality, identification of problems or shortcomings in the delivery of care, the design of activities to overcome these deficiencies and follow-up monitoring to ensure effectiveness of corrective steps.
Box 4.2 Synthesis of emerging screening criteria proposed since 1968
- The screening programme should respond to a recognized need
- The objectives of screening should be defined at the outset
- There should be a defined target population
- There should be scientific evidence of screening programme effectiveness
- The programme should integrate education, testing, clinical services and programme management
- There should be quality assurance, with mechanisms to minimize the potential risks of screening
- The programme should ensure informed choice, confidentiality and respect for autonomy
- The programme should promote equity and access to screening for the entire target population
- Programme evaluation should be planned from the outset
- The overall benefits of screening should outweigh the harm
Source: Andermann et al. 2008 (3).
In agreement with the WHO criteria, the Council of the European Union has recommended cancer screening with a systematic population-based approach and quality assurance at all appropriate levels (5). Screening programmes are recommended for breast, cervical and colorectal cancers in agreement with evidence-based guidelines. According to the final report on the implementation of the Council recommendation on cancer screening, most EU countries are planning, piloting or implementing population-based screening programmes for breast, cervical and colorectal cancers (6).
However, there are deficiencies in utilization of some programmes (e.g. because of very low attendance rate), indicating ineffectiveness and likely social inequalities, and in the monitoring and evaluation capabilities required for comprehensive quality assurance.
The quality-assured implementation of cancer screening for the above three cancers involves careful planning and piloting, and scaling up from pilot to sustainable full-scale national rollout based on social and service provider acceptance (7,8).
Fig. 4.1 illustrates various steps and phases of the process. Formulation of a screening policy proposal requires evidence on the effects of screening, disease burden, quality-assured testing and treatment and primary prevention possibilities. Adequate performance must be verified from the beginning, allowing the detection and correction of potential undesirable trends. When problems are identified, the activity needs improvement, reorganization or even discontinuation (Fig. 4.1) (2,7,9).
Modifications of existing programmes are also needed to reflect developments in screening, diagnostic and treatment methods, or because of developments in complementary primary prevention (e.g. HPV vaccination). Systematic quality assurance needs continuous well-integrated interplay between policy-making, evaluation and implementation.
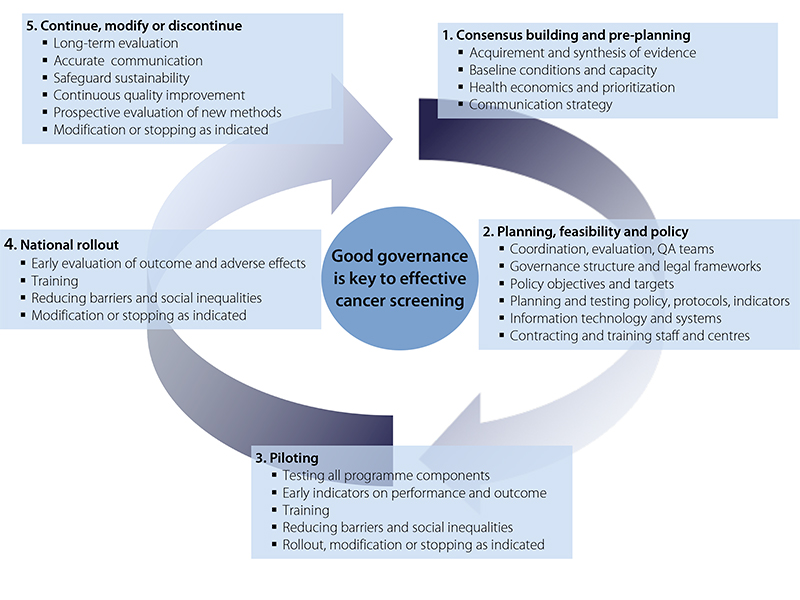
Fig. 4.1 Examples of tasks of organization, evaluation and governance in different phases of implementation and quality improvement of a cancer screening programme.
The purpose of the chapter is to produce further advice and guidance for the development and implementation of cancer screening in the EU Member States in accordance with the EU Council recommendation and the current European quality assurance guidelines. Given their scale, population-based programmes need solid governance structures. Appropriate legal frameworks are required to run and monitor organized programmes and evaluate their outcomes; in addition, human and financial resources are needed for assuring the appropriate organization and quality control (5). The chapter presents 12 recommendations covering the spectrum of themes relevant for initiating and running population-based cancer screening programmes: governance of cancer screening, organizational requirements, the need for integrated evaluation and the approach and considerations for potential new cancer screening programmes. Solid screening governance is necessary throughout the process illustrated in Fig. 4.1. The organizational requirements deal with key issues, particularly for building capacity and capabilities in phases 2 through 4 outlined in Fig. 4.1. Integrated evaluation is necessary to inform actions at each step of the cycle. Before exploring these themes, the methodology and evidence base are described.
Methods
Evidence on efficacy and effectiveness of cancer screening was drawn from recent systematic reviews, and European quality assurance guidelines with ratings of evidence were utilized. The information was supplemented and updated also with conventional literature searches using PubMed. The most relevant guidelines and systematic reviews used were: European guidelines for quality assurance on breast (10,11), cervix (8,12) and colorectal (13) cancer screening; WHO position paper on mammography screening (14); IARC Handbook on Breast Cancer Screening (15); Cochrane review on colorectal cancer screening by test methods (16,17), supplemented with a more recent meta-analysis on flexible sigmoidoscopy screening (18); and the European Code against Cancer’s scientific justification on recommendations for cancer screening (19). Specific literature searches were used for potential new screening programmes for prostate, lung, gastric and ovarian cancers.
Current evidence for prostate and gastric cancer screening was discussed at the respective consensus meetings with international experts. Status reports on the implementation of cancer screening were available from surveys: IARC 2008 (20), EUNICE (21), EuroScreen (22), EU Joint Research Centre (23,24), IARC ongoing (6) and the CanCon cervical cancer screening working group (supplemental information available at the CanCon web site, http://www.cancercontrol.eu/). Earlier documents on the concepts and further recommendations on the implementation of cancer screening as a part of cancer control policies were also reviewed: EPAAC documents on planning for cancer control strategies with a section on cancer screening, the curriculum report ESSM (25) and materials EUROCOURSE (26) and the European Science Advisory Network for Health documents and reports (7,27).
Supplementary data on current implementation status of cancer screening programmes in individual Member States, including further organizational details, resources, governance and decision-making processes, legal frameworks, quality assurance and quality management systems, were obtained through the partners and experts participating in the working group meetings for the Work Package on Cancer Screening, held between May 2015 and February 2016. This data collection process obtained information not published in scientific papers.
Information was requested on particular achievements as well as bottlenecks and barriers. In the working group meetings, suggestions of relevant to pics regarding policy-making were collected. Recommendations of the guide chapter were drafted by the authors of the chapter and delivered for comments and review within the working group. In connection with the Cervical Cancer Screening Working Group, a survey on governance and legal frameworks was performed for all 35 EU and European Fair Trade Association countries and devolved nations of the United Kingdom (supplemental information available at the CanCon web site; http://www.cancercontrol.eu/).
Information on this survey in this document is based on answers from the 33 countries that had responded by September 2016. In the formulation of the general recommendations on governance structures and functions, the publicly available protocols from the United Kingdom, Norway and Sweden, specifically developed to deal with issues concerning national screening programmes, were consulted (2,28,29).
Governance of cancer screening
Governance and decision-making processes are at the core of well-functioning cancer screening (Fig. 4.1). Governance is here to be understood in the conceptual framework of stewardship as elaborated by WHO (30–32). This implies that the ministries in charge of health assume the ultimate responsibility for the management of the national resources to the health benefit of their entire population by directing the establishment of as good and fair a health system as possible and by promoting health aspects in all policies (33,34).
Governance in the health sector refers, therefore, to a wide range of steering and rule-making functions carried out by governments and decisionmakers to achieve and develop national health policy objectives. This involves policy development and implementation, detecting and correcting undesirable trends, influencing or regulating health care funders and providers, and establishing accountability mechanisms (e.g. by monitoring and evaluating health system performance). While the scope for governance is usually greatest at the national or legislative level, it also covers the steering role of regional and local authorities, and involvement of stakeholders at all levels is essential.
For screening in particular, the national policy-making and governance structure should ensure a thorough and professionally sound procedure for the assessment and introduction of new national screening programmes and for major modifications to, and if necessary the discontinuation of, those programmes. Appropriate legal provisions must be in place and the governance structure should ensure follow-up, quality assurance and evaluation of existing programmes. These requirements are common to all cancer screening programmes and, therefore, governance according to a common general template can be recommended. In what follows, we describe the policy-making and governance structures, legal framework and quality assurance mechanisms that are needed for well-functioning screening programmes.
Governance structures, policy-making and stakeholder support
Many Member States have found it challenging to implement sustainable screening programmes that fulfil the potential for equitable cancer prevention as recommended by the Council of the European Union in 2003 (20,35–37). The lack of adequate governance and policy-making structures to ensure infrastructure and organizational support appears to be a key barrier (36,37). There are examples where screening programme implementation has failed to produce expected benefits, or has suffered from severe impediments, because necessary organizational, legal, logistic or financial frameworks were not adequately addressed in advance (37,38).
In these cases, a more structured approach to governance and decision-making would be beneficial (39). 6 European Guide on Quality Improvement in Comprehensive Cancer Control Fig. 4.2 shows what a governance structure can look like covering the key tasks: (i) policy-making, here embodied as a national screening board advising the ministry; (ii) supervision by cancer sitespecific steering boards; (iii) management, here performed by one national or several regional management team(s); and (iv) feedback from screening providers and the scientific community through advisory boards or similar organs. All these functions and designated responsibilities should be covered in the governance structure, while allowing considerable adaptation according to the local setting and circumstances. Each of the elements will be looked at in detail below.
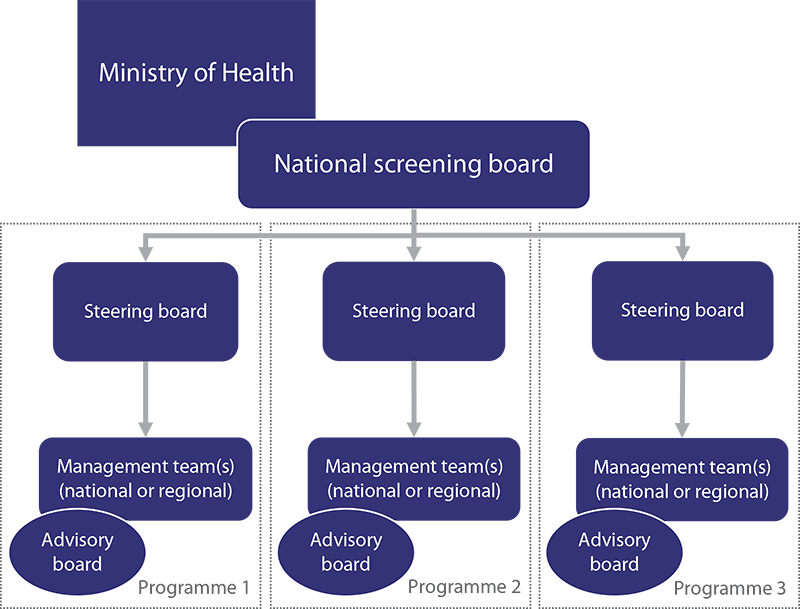
Fig. 4.2 Organizational chart of an example national governance structure
In a small number of countries with successful population-based screening programmes, decisionmaking and governance structures, tasks and procedures have been formally defined (2,28,29,40). The policy-making and screening governance in the Netherlands provides a good example where the Health Council, which produces scientific advice on health policy, both unsolicited and solicited by the ministry in charge of health, includes a permanent Committee on Population Screening (corresponding to a national screening board in Fig. 4.2, below) (40). The resulting detailed advice includes provisions for the requirements for successful implementation (41).
The Centre of Population Screening of the Dutch National Institute for Public Health and the Environment carries out feasibility studies and finances, directs and coordinates implemented programmes. Where an effective monitoring system is in place, the process, costs and effects of new policies, introduced on a small scale under controlled circumstances, can be easily measured and tailored if necessary before general roll-out. Well-developed policy-making and governance structures should also promote the allocation of necessary resources for RCTs and for piloting programmes or modifications as randomized health service studies (42–44). An interim analysis of the results of a survey conducted as part of the activities of CanCon suggests that many European countries lack several components of the governance structure of cervical cancer screening (Fig. 4.3).
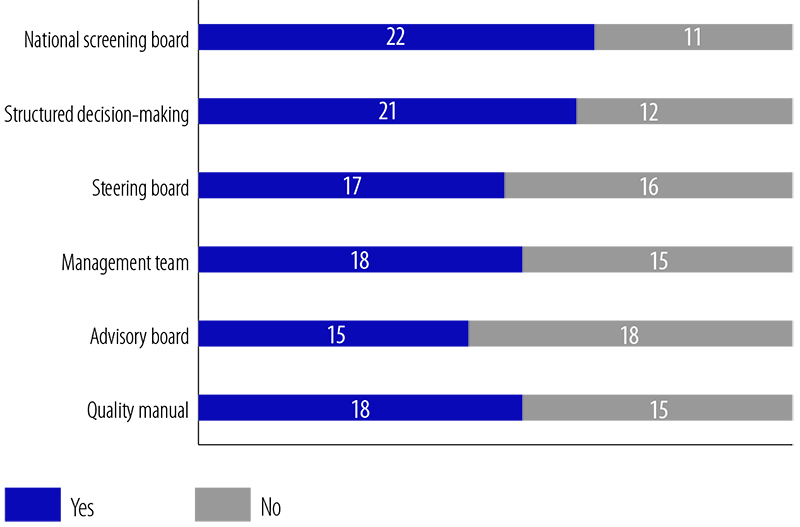
Fig. 4.3 Governance of cervical cancer screening in Europe
Note: The figure summarizes the number of countries reporting the presence of each governance structure component (see the text for definitions of each component addressed).
The EU Council recommendations and the EU guidelines set a common framework for qualityassured screening. Each country has to assess how screening according to these principles can feasibly be organized within their health system and should identify and remedy gaps in the available resources and infrastructure. In countries where domestic experience of effective, wellorganized population-based cancer screening is lacking, international collaboration with expert units experienced in coordinating and evaluating screening programmes can be useful or necessary in the planning and piloting phases (25,45). It is not necessary for each Member State to perform all generic health technology assessments independently; collaboration could save significant resources and avoid duplication, for example on choice of test technology. European-level data repositories and the production of standardized quality indicators would also be desirable in that it would promote comparability of programmes, compliance with guidelines and quality of screening across the EU.
The importance of political commitment
The quality of political decision-making is critical for any public health activity. In the case of cancer screening, this includes a long-term commitment to follow guidelines and to assure quality at all stages of the screening chain (7). Appropriate synthesis of evidence and assessment of baseline conditions such as disease burden and existing and potential treatment capacity are of utmost importance from the outset. Commitment to invest implies expected returns in terms of deaths prevented, quality-adjusted life-years (QALYs) gained and/or downstream treatment costs saved. Political decision-making without commitment to assure quality of the screening process may be detrimental to trust in cancer screening, both in the target population and among professionals, and should be strongly discouraged. A regional or national parliament agreement may be needed in order to assure the long-term commitment.
A national screening board to advise decision-makers on national screening programmes
A designated national screening board, or other such competent entity, should be responsible for advice on policies and decision-making regarding new population-based screening programmes or modifications to existing programmes (Fig. 4.2). The process should be structured and defined in a transparent procedure based on clear, evidence-based criteria to ensure that a proposed new or modified screening programme is able to reach an optimal balance between benefit, harm and costs (by measures capturing the relevant health impacts to a sufficient degree, such as cost per QALY gained). The board should ensure that the necessary organizational, logistic, legal and financial frameworks exist or can be developed. Defining institutional responsibilities, collaboration between the key institutes and consultation with relevant stakeholders allows benefit from existing expertise and broad support and commitment. The decision should be reviewed before each step in the implementation process: feasibility testing, piloting and full-scale roll-out of service screening (Fig. 4.1) (7). A multistep decision-making process is necessary because the performance and outcomes of the proposed screening programme may differ significantly from those demonstrated in controlled trials, as well as from other service settings, and the full impact of these differences may not be evident in advance (43).
Programme-specific steering boards: oversight and sustainability
Once a decision to implement a screening programme has been politically ratified, a programmespecific steering board is required. The steering board oversees both the implementation phases and the sustainability and continuous incremental improvement through the quality assurance processes of the established programme. The steering board should shoulder the executive professional responsibility for the performance, quality assurance and evaluation of the screening programme, including the continuous assessment of the test methods and procedures, and the financial, ethical and legal frameworks. It officially sets and maintains the overall goals of the screening programme. It also ensures that the means and mechanisms are in place to monitor and achieve those goals. It is the forum for resolving political, legal, organizational, technical, cost and management issues that have not been resolved elsewhere. To fulfil its tasks, the steering board must have access to both political and high-level administrative decision-makers, and it must be representative of the key stakeholders, including programme management. The steering board may also decide to submit a proposal for a major modification or cessation of the screening programme under its jurisdiction to the national screening board. The steering board should convene regularly, several times a year.
Programme-specific management teams: execution and reporting
Successful implementation and a sustainable screening programme with integrated quality assurance and the capacity for continuous quality improvement requires a competent management team running the programme on a day-to-day basis at the national or regional level, with a clear mandate from the steering board and the necessary resources to fulfil its responsibilities (see below). These responsibilities include coordination or supervision of all steps in the screening process from identification of the target population to surveillance after treatment of screen-detected cases. It further includes the development and dissemination of information material, collection and validation of monitoring data, regular compilation and linkage with other relevant registers for reporting of performance and outcome of screening, coordination of quality assurance activities, and the further development and continuous quality improvement of the screening programme according to directions and frameworks given by the steering board. Periodic formal programme evaluation may be tasked to an independent unit in order to avoid self-assessment by the management team. Some responsibilities may feasibly be delegated to the regional and local levels along with the adequate mandates and resources. In federated and larger countries, regions may have their own management teams, but policy should be formulated at the national level. Programme evaluation should also have a national scope.
Advisory boards: linking management and providers
Successful programme management depends on good communication with all screening service providers, and access to their expertise (25). A multidisciplinary advisory board or forum can fulfil these functions by providing representation for the professional groups and institutions that screening delivery depends upon, facilitating the flow of information of issues of current import between management and the screening service providers and advocacy groups, and sharing information with academic and professional societies and institutions. The advisory board to the Norwegian screening programme for cervical cancer, as an example, is a multidisciplinary board including representatives from professional bodies (pathology, clinical cytology, gynaecology, gyno-oncology, general medicine, medical laboratory technology, epidemiology, microbiology) in addition to the Cancer Society and the National Reference laboratory for HPV (46). The appointment of one advisory board member as responsible for equity issues in the screening programme is recommended. Based on the cooperation of the advisory board and the management team, it is advisable to produce a programme-specific quality manual that describes the procedures and protocols that fulfil the quality requirements in that particular programme (47). The local quality manual should be in accordance with the relevant European quality assurance guidelines. Only a handful of countries in Europe have screening programmes with all or most of the governance structure components described in this section. A survey of governance structures for cervical cancer screening in 33 countries showed that countries are often lacking one or more governance component (Fig. 4.3).
Recommendation 4.1 Successful evidence-based cancer screening needs a competent, multidisciplinary and transparent governance structure with political, financial and stakeholder support.
Legal framework for population-based cancer screening
Population-based screening is a complex undertaking that needs careful coordination and monitoring of performance and outcome. In most cases, a legal framework needs to be developed that is designed to run the health services and to regulate the comprehensive information systems required to manage and to ensure the quality of population-based screening programmes. The legal framework should provide regulation of patient rights, consent requirements, institutional responsibilities, financing and tendering, personal data safety, electronic health records, tissue sampling and biobanking, population and cancer registration, and scientific research and development (12,48). The legal framework and information systems for population-based screening must secure an adequate balance between fundamental rights of privacy and access to effective, safe, high-quality and cost-efficient health services. Confidentiality of personally identifiable information on health status must be protected while fulfilling the duty to demonstrate and optimize health benefits and minimize negative effects and costs of screening.
Registration and linkage
Effective screening management necessitates a legal mandate to register centrally all screening, diagnostic and treatment activity with a personal identifier, including negative test results, and to cover both programme-initiated and opportunistic testing. The registration must be sufficiently detailed, of high quality and complete (8,11), which precludes active consent requirements for registration. The crucial requirement for successful implementation of quality-assured population-based screening is the possibility for linkage of at least population (target group identification), cancer and cause of death (outcome information) with and screening registers (performance information) (12,49). This requires the building of population-based cancer registries where such registries do not yet exist (26). An audit of the screening and treatment histories of all cancer cases arising in the population covered by the screening programme, and comparison of these screening histories with those of population-based controls, provides a possibility of evaluating the effectiveness of the screening programme and yields crucial information on its strengths and weaknesses. Such audits allow rational decisions to be made on modifications to screening policy and protocols, enable repeated incremental improvements to effectiveness and the prioritization of quality assurance efforts. Linkage with other registers such as vaccination and hospital episode registers can also be useful or required for adequate management, monitoring and evaluation. As for registration, such linkages should not be based on active consent. Evaluation based only on consenting individuals are likely to be biased and misleading (50). However, appropriate data protection safeguards should be in place to ensure privacy.
Invitation and fail-safe monitoring
A population-based screening programme relies on the identification and personal invitation of all those in the defined target population. There must also be fail-safe monitoring to ensure adequate management of those screening positive. Consequently, those managing the screening programme must have access to a current population register with contact information and unique personal identifiers for correct linkage to screening databases and other relevant data sources. Depending on policy, invitations are sent based on a combination of age and screening or medical history. Management teams must, therefore, have the legal mandate to contact people directly based on their screening history with invitations and reminders, and to keep administrative records of this activity.
Current status of the legal framework for screening in Europe
The lack of an adequate legal framework has been recognized as a major obstacle to effective screening programme implementation in several settings. Nevertheless, data collection and linkage must be in agreement with legal regulations. When legal barriers impede crucial data exchange operations, adaptations of local law may be required. According to results of the survey conducted in connection with the CanCon Cervical Cancer Screening Working Group, there are still significant barriers to many essential functions of population-based cancer screening in Europe (Fig. 4.4).
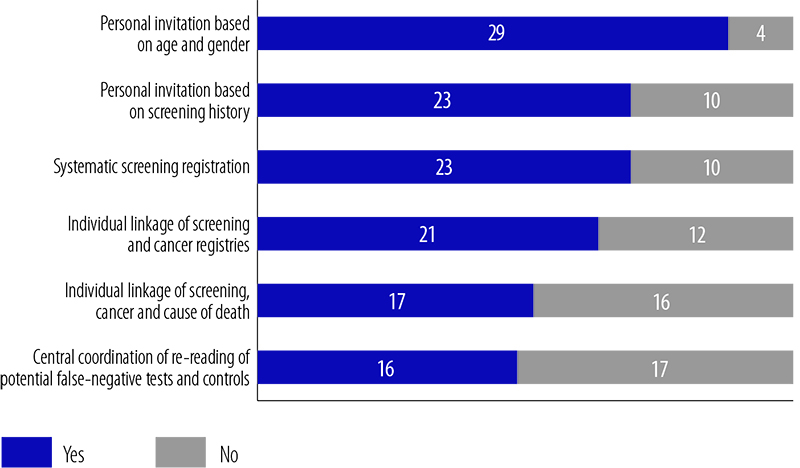
Fig. 4.4 Legal frameworks for cervical cancer screening in 33 European countries
Note: The figure summarizes the number of countries reporting that their legal framework allows (prescribes in the case of systematic screening registration) each of six operational functions of the screening programme.
Recommendation 4.2
The legal code in a country should provide a specific framework for population-based cancer screening, enabling as a minimum the following basic functions: personal invitation, mandatory notification and central registration of complete screening and outcome data, individual linkage to cancer and cause of death registries for appropriate quality assurance, including audits.
Resources for quality assurance
Population-based programmes with appropriate quality assurance have not been fully implemented in all Member States since adoption of the recommendation on cancer screening by the Council of the European Union (5). In certain countries or regions, no such programmes exist (6,20,23,25). Integrated quality assurance has proved to be necessary to secure the potential benefits of population-based screening and limit the associated harms and costs. However, earmarked resources are needed for this activity, resources that are not always budgeted in the planning phases leading to implementation of screening. It is crucial to realize that adequate
resourcing is a prerequisite for a screening programme, and that it may be better to limit the scope of the screening programme (such as number of lifetime tests) rather than neglect quality assurance if resources are scarce.
Wide variation in practices and effectiveness is observed throughout the EU, and inefficient opportunistic activities still dominate screening in several countries. A recent review on quality assurance standards and programmes in the cervical cancer screening programmes in Europe showed that organized efforts for quality assurance, including auditing, monitoring and evaluation, were carried out to a differing extent and were not standardized (Annex 4.1) (24). Most countries found it hard to estimate the costs associated with launching and operating the organized programme. Similar systematic information on the routine audit practices of breast and colorectal screening programmes is not currently available. Nationwide, population-based registration of breast, cervical and colorectal cancer is not yet feasible in all EU Member States. In 2012, cancer registry coverage of the combined European populations was somewhat more than 60%, and systematic evaluation of cancer control and quality of care remained modest, except in a few dedicated cancer registries. Evaluation of mass screening programmes was supported more or less routinely by only 44% of cancer registries (51)
Considerable challenges, therefore, remain to bolster health equity. The lack of comprehensive quality assurance in settings relying on opportunistic testing, or even in population-based services, generally results in a less favourable benefit-harm balance compared with screening with integrated quality assurance (20,36).
Components included in comprehensive quality assurance are listed in Box 4.3. Based on the European recommendations (8,10–13), systematic quality assurance requires defined protocols for standard procedures and quality management throughout the diagnostic and patient management services within the programme. In addition, a substantial proportion of the resources in quality assurance are required for well-organized information systems that support the aims of screening registries and population-based cancer registries (10,52). This infrastructure is necessary to systematically audit the programme policies and services, as recommended in the guidelines (10,12). Adherence to these general principles and recommendations on systematic quality assurance is an ethical imperative to assure that the screening services delivered to the
population are appropriate (49). Quality assurance also includes timely, prospective evaluations of modifications of existing programmes and for piloting new programmes (8).
Box 4.3 Functions and budgetary items for the quality assurance allocation of 10–20% of total screening programme expenditure, in accordance with the European guidelines for quality assurance in cancer screening
- Development and maintenance of well-organized information systems
- Clinical and diagnostic quality assurance and quality management
- Development of population-based cancer registration and other databases for adequate monitoring of the burden of disease and the outcomes of screening
- Development, implementation and enforcement of a quality manual based on European and national standards
- Reporting of performance and outcome indicators based on European and national standards
- Retrospective evaluation of the effectiveness of the programme and its components
- Prospective evaluation and introduction of new screening methods, policies and organizational models
Recommendation 4.3
Successful implementation of effective cancer screening programmes requires significant resources for quality assurance that is 10-20% of the estimated total expenditure of a full-scale programme.
Organizational requirements
Adequate organization and coordination of screening are important at all stages of programme development from preplanning and feasibility testing to implementation piloting, roll-out and continuous improvement.
Organization and infrastructure
A programme should be thoroughly preplanned for the target ages, screening interval and tests used to identify preclinical disease. Appropriate synthesis of evidence on effectiveness, adverse effects, health-economic aspects, in combination with information on the burden of disease, is essential background information for these tasks. In this phase there should be data also for estimation of the invitational population size per year, planning for feasible schemes to cover the target population often enough (interval between invitations) and plans on how to reach a high uptake of the primary test and guarantee fully quality-assured management services ready at the time of starting.
Population-based cancer screening has infrastructure requirements that need to be verified or developed before starting to screen. First, the target population (age, region, gender) has to be individually identified to allow a call and recall system. For follow-up of screening outcomes, population-based registration of both cancer and screening is needed. Additionally, time and cause of death has to be individually linked with screening invitation information for outcome evaluation purposes. The development of a comprehensive quality assurance plan and manual needs to precede the start of screening activity. Planning includes verification of adequate capacity through the whole screening chain from individual identification of persons to be invited to treatment and follow-up of screen-detected lesions.
A new programme and all its components, or new procedures in an existing programme, should be feasibility tested and piloted in a controlled fashion before national roll-out (Fig. 4.1). Initial training and development of competence can be focused on a developing national screening reference centre or area, where feasibility testing and piloting can be based, and where subsequent training needs for the roll-out phase can be satisfied. The invitational procedures with call and recall, acceptance of testing, communication with the screened person, delivery of further investigations (e.g. diagnostics and treatment), costs and other details not yet known at launch may provide challenges. For example, the uptake of screening may depend on the premises where samples are taken, opening hours, public traffic, personnel (women for breast or cervical cancer screening), among many other factors.
After the piloting phase, the programme can be rolled out after modifications and corrections deemed necessary based on pilot evaluation. The full implementation of the programme may take several years to achieve coverage and ensure optimal function through the screening chain. A gradual build up is usually needed to ensure practical resources, for example colonoscopy services for those who are positive for faecal occult blood test. Integrated comprehensive quality assurance allows for further incremental improvement in a continuous quality cycle. A high level of organization with solid governance and coordinating functions also give better opportunities to stop ineffective or harmful activities in a controlled fashion. If existing screening does not fulfil quality requirements, the decision must be either to reorganize by following EU guidelines or ultimately to stop the ineffective programme. Continuation of an ineffective programme is unacceptable. Modifications from ongoing opportunistic testing (either self-selection or general recommendations as opposed to invitation based) towards population-based programmes are encouraged.
Recommendation 4.4
Implementation of population-based screening should be a carefully managed multistep process through the phases of coordinated planning, piloting, roll-out and continuous improvement.
Coordination
Following the political decision with associated budget allocations to start implementing a population-based cancer screening programme, and formulation of its goals and frameworks, the first step is to establish coordination and allocate institutional responsibilities. The institution housing the management unit should receive a clear mandate and resources to manage the entire process of programme implementation depicted in Fig. 4.1. The management unit also has to prepare the budget details through all phases, including the resources required for quality assurance, programme management and staff training. The work necessitates close collaboration with authorities and all stakeholders, preferably within a well-defined and mandated governance structure (Fig. 4.2). The mandate may also require changes in national legislation to ensure that it does not contradict effective implementation. Considerable autonomy to take organizational decisions must be allowed for coordination.
Multidisciplinary management and evaluation teams
It is essential that a screening programme is managed by specialists with adequate knowledge and training in the subject areas of cancer screening. Specific training possibilities in the EU are available. Experience from other EU countries could be helpful; experts from other countries should be involved as consultants if local expertise is unsatisfactory. Professional expertise should be utilized from the planning phase (development of standards and quality indicators) and throughout the implementation and for continuous evaluation. The professional and organizational management structure must be equipped with the competence and the mandate to control the quality of the entire screening process. Recent European guidelines and available European expertise should be consulted regarding questions on efficacy and effectiveness of new technologies.
It may not be necessary to complete a national health technology assessment on questions related to new tests or diagnostic/therapeutic procedures if thorough international evaluations already exist. In that case national health technology assessment agencies could focus on questions related to local implementation and costs. Registration and information technology systems The formation of a centralized data registration system for quality assurance is critical for the success of a programme. The format of the data follows standards developed by professionals and based on the European quality assurance guidelines.
Although linkage to the screening procedure reimbursement system is desired, it is essential that the system is not limited to invitation and procedure reimbursement but also covers performance and outcome of the screening programme. The requirements for continuous quality assurance should be considered early on and incorporated when designing the comprehensive information technology system that covers the entire screening process, including the quality of treatment of detected lesions. The established quality assurance system should also be used for procedures outside the screening programme. In most EU countries, the screening data platform has not been embedded in a comprehensive clinical health (e-health) data system; however, this would be highly recommended.
Recommendation 4.5 The mandate and resources for screening coordination and training, and for the electronic information systems necessary for quality assurance and incremental improvement, must be secured before starting the population-based screening service.Integrated evaluation, part I
Linkage and indicators for quality and effectiveness
The Council of the European Union recognized that quality screening includes analysis of the process and outcome of the screening, and that this analysis is facilitated if the screening database can be linked to cancer and mortality databases (5). The European guidelines for quality assurance in breast, cervical and colorectal screening (10,12,13) all emphasize data linkage between screening and cancer registries; implementation has, however, been limited throughout Europe (54). In this context, the FP7 European project EUROCOURSE formulated a set of recommendations for data interfaces between screening programmes and cancer registries as well as with other information sources (26). A set of performance indicators has been generated separately for each screening programme for comparative monitoring at the European level, and the importance of linking the screening data not only with cancer registry data but also with other registries of interest (population, cause of death, diagnostic and treatment registries and, more recently, HPV vaccination and biomaterial registries) has been emphasized (26,48). While the linkage between cancer registries and mortality databases has been established in most European cancer registries, linking the data from national screening databases and cancer registries still poses a significant issue in some (Fig. 4.4).
The Council of the European Union recommendation mentions a need for monitoring specific performance indicators, without detailing the nature of these indicators. The specific guidelines discussed above describe these indicators thoroughly and set the desired levels. For example, in breast cancer screening the desired invitational coverage is 100%, the attendance rate over 75%, the rate of recalled less than 3% and first year sensitivity over 70%. The other approach to assessing performance is the rate of false positives (recalled women whose examinations end with a negative result) and the overdiagnosis rate (breast cancers that would not have come to clinical attention were it not for screening). The estimates from routine screening for the latter vary considerably (1–54%), although it is reduced to 1–10% when adjusted correctly for lead time (55).
Recommendation 4.6
To secure the benefits of screening, routine linkage between the registries containing relevant data for defining the population, performance and outcome is essential and can be considered an ethical requirement of screening.
----
Integrated evaluation chapter continues in part II.
See part II of Cancer screening chapter