Integrated cancer control, part I
The case for comprehensive cancer care networks (CCCN)
This is a part I of chapter Integrated cancer control (Cancon work package 6) of the Guide. See part II of the chapter.
See the full Guide and other chapters as pdf's.
Cancon Guide is the main delivery of the joint action.
Contents of Integrated cancer control part I:
Introduction
Methods
Results
Building a CCCN
Operation of a CCCN
Integrated cancer control part II:
A real-life, real-time example of creating a CCCN (Czechia)
Discussion
Conclusions
References
Introduction
The rapidity of change in the cancer landscape must be considered when pursuing the objective of cancer control in the 21st century. First, the cancer incidence in Europe is continuing to increase, in large part for demographic reasons as the age distribution of the population has shifted upwards.
Second, improvements in technology make cancer diagnosis possible earlier, but sometimes at the cost of false diagnosis or overdiagnosis. Third, management protocols are changing all the time, not only through availability of new and better targeted drugs but also through immunotherapy approaches finally becoming successful and advances in imaging techniques, surgery, radiotherapy and other locoregional approaches. Many of these advances come at a high cost, as they may require providing very expensive drugs or substantial investment in new equipment using more advanced technology. Fourth, advances in understanding the molecular basis of cancer have revealed that cancer is much more heterogeneous than it was thought to be hitherto: in the end, each tumour is defined by the set of (somatic) mutations that have caused it and this has direct implications on how that tumour ought to be managed (i.e. personalized cancer medicine).
Last but not least, there is increased recognition that supportive treatments, ranging from palliative care to psychosocial-oncology, must be part and parcel of the management of cancer patients. In view of these changes, it is imperative to consider whether and what actions are needed to improve how cancer control is structured, for two main reasons: (i) cancer control can be improved, but it should be improved in a cost-effective way and (ii) improvements in outcomes must not be confined to a select few but should be for the benefit of all those who are affected. Outcomes must be measured at the population level, with the aim of reducing present-day inequalities (1), and ideally eliminating them. Since the landmark Calman-Hike report in the mid-1990s (2), networking and formalized collaboration between health care providers is increasingly recognized as an option for delivering cancer services (3,4).
Currently, cancer control is organized in different ways in different countries, not uncommonly even within different regions of the same country. Prevention, screening programmes and management of patients may be carried out by the same institution or by different institutions. Various existing cancer care patterns may be influenced by history, by population size and density, by the geographic distribution of the population, by the health system structure and resources available, or by past and present policy decisions. As long as a system functions well, provides optimal outcomes and is cost-effective, there is no reason to change its basic structure; if different approaches serve the purpose of cancer control, there is no reason, in principle, to impose the same modalities everywhere. However, in most systems there is room for improvement.
Today in Europe many cancer patients are treated in general hospitals, whether public or private, and/or in institutions specialized in cancer management, including those that have become known as comprehensive cancer centres. A consistent and broadly applicable definition of a comprehensive cancer centre does not exist, but the Organization of European Cancer Institutes in its voluntary-based accreditation procedure lays emphasis on a wide range of elements, including infrastructure for cancer care, human resources, clinical care activities, research activities, education and institutional structure (5). In the United States, the National Cancer Institute (6) recognizes that a comprehensive cancer centre is a centre that carries out “cancer research and provide services directly to cancer patients. Scientists and doctors at these centres do basic laboratory research and clinical trials, and they study the patterns, causes, and control of cancer in groups of people. Also, they take part in multicentre clinical trials, which enrol patients from many parts of the country. Comprehensive Cancer Centres also give cancer information to health care professionals and
the public.” A comprehensive cancer centre implies a concentration in one location of qualified oncologydedicated staff; volumes of patients sufficiently large to produce economies of scale; adequate numbers of patients with less common tumours that require special expertise; ongoing opportunities for keeping all personnel up to date; ability to design and to run clinical trials; expertise in epidemiology, oncology and cancer research in various areas; and facilities for data management. Importantly, the superiority of such centres in terms of treatment outcomes (or of specialized similar entities embedded in large hospitals or specialized units) has been well documented (7,8).
This chapter explores a model of integrated cancer control that reconciles the expertise of highvolume specialized referral centres with the greater accessibility of general hospitals, other health care institutions (e.g. imaging centres, community care centres) and primary care professionals (PCPs, e.g. GPs, home nurses and others). Networking models are attractive in principle because, by fostering communication and collaboration, they can both draw on the experience and the abilities of the constituent units, thus implementing synergies, and significantly reduce geographical inequalities by having multiple entry points and by offering cancer control services to the entire population living within a certain area.
Here we outline the features of one particular type of highly integrated network, the Comprehensive Cancer Care Network (CCCN). This chapter describes how CCCNs can be planned and established, how they work and what purpose they serve. It also includes policy recommendations that may prove useful for countries and stakeholders active in cancer networks.
Since different people/agencies may mean different things by the word network, here we have agreed, after extensive discussions and deliberations, on the following definition of CCCN (Box 5.1)
Box 5.1 Definition of a CCCN as used in this volume
- A CCCN consists of multiple units belonging to different institutions dedicated to research, prevention, diagnosis, treatment, follow-up, supportive and palliative care and rehabilitation for the benefit of cancer patients and cancer survivors. The key elements defining a CCCN are illustrated in Fig. 5.1.
-
These units a) interact and have a formal agreement to work together in a programmatic and structured way with common governance, in order to pursue their goals more effectively and efficiently through collective synergies.
- Within the CCCN the care of patients is the responsibility of interprofessional teams that are multidisciplinary and tumour specific. Each team or tumour management group works together for the benefit of patients with that particular type of tumour.
- Within the CCCN all units work together and adopt uniform standards of care for cancer-specific pathways that are binding for the entire network.
- The CCCN promotes a uniform system of quality assurance; and a unified informatics system for optimal exchange of information.
- The objective of a CCCN is to provide comprehensive cancer care to all the people living in a certain geographic area, thus pursuing equality and the improvement of outcomes and quality.
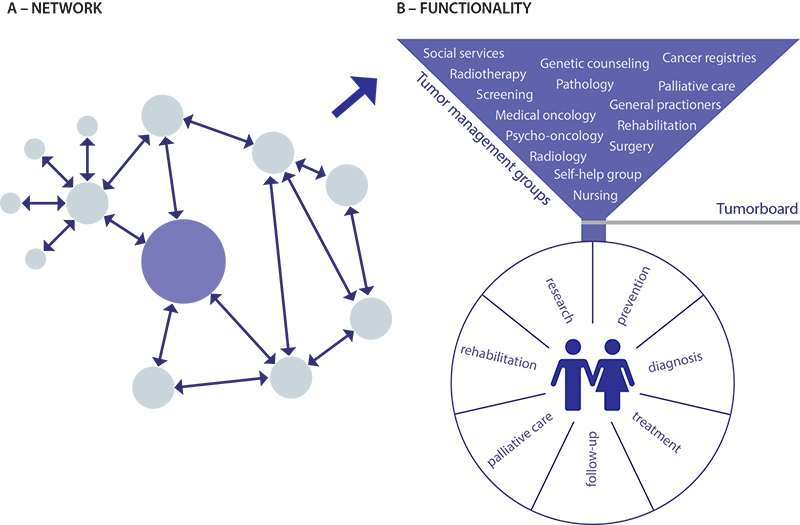
Fig. 5.1 The key elements defining a CCCN: (A) example of a network; (B) tumour management groups
Legend
A – Network: The dots represent units/institutions (e.g. primary care, community hospitals, university hospitals, psychosocial counselling etc.) dedicated to research, prevention, diagnosis, treatment, follow-up, supportive and palliative care and rehabilitation, which work together as a CCCN in a structured way with a common governance
B – Functionality: The tumor management groups within the CCCN are inter-professional, multidisciplinary and tumor-specific; with the objective to provide comprehensive cancer care to all the people living in a certain geographic area
Notes: The example of a network (A) has arrows to indicate the flow of patients, expertise, data and so on between networking institutions of different sizes, with different roles and at different levels in the health system. These work together in a structured way with a common governance.
The dots indicate units/institutions (e.g. primary care, community hospitals, university hospitals, psychosocial counselling) dedicated to research, prevention, diagnosis, treatment, follow-up, supportive and palliative care and rehabilitation. In tumour management groups (B), care-related activities are interprofessional, multidisciplinary and tumour specific. The objective is to provide comprehensive cancer care to the entire population within the catchment area.
Methods
Surveys
As a preliminary to proposing a specific type of network as a possible model of cancer care organization, we thought it was appropriate to survey what already existed across Europe. A survey collected specific items of information from representatives of EU health authorities, cancer societies, directors of comprehensive cancer centres, cancer registry directors and others. Since a pilot CCCN study is currently ongoing in the Czechia as part of CanCon Work Package 6 (see below), this country was not formally surveyed. Of the remaining 27 EU countries 25 responded (see supplemental information at www.cancercontrol.eu).
A survey was also carried out to assess how rare cancers are cared for in various countries, as a collaboration of RARECAREnet with CanCon; more specifically, the survey aimed to understand how centres of expertise for rare cancers are being identified in EU countries and how they actually operate. The surveys consisted of semi-structured interviews with international experts who have a significant role in governance and organization of cancer networks in their respective countries (supplemental information provided at www.cancercontrol.eu). This served to obtain information on the decision-making process that led to the establishment of a specific cancer care model.
Literature search strategy
Three strategies were employed for the literature search.
-
Relevant literature published in peer-reviewed journals and/or grey literature was identified through systematically searching of medical databases such as PubMed (including Medline, Medical Literature analysis and Retrieval System Online), Google database, Scopus and Web of Science until November, 2015; the exception was “Palliative care” where the literature search was conducted until March, 2016. Boolean operators “AND” and “OR” were used as deemed relevant (supplemental information provided at www.cancercontrol.eu).
-
Cancer network, government and regional web sites were also searched for relevant reports. Articles related to organizational structures in the form of networks (i.e. CCCNs or similar) in Europe were of particular interest and there was a focus on articles discussing organizational structure of CCCNs in Europe.
-
A basic exploratory online database search combining the search terms “travel distance” AND “cancer” (no further restrictions) was used to examine territorial inequalities in access to cancer care.
For describing CCCN characteristics and functions, a search of PubMed was conducted using the following keywords and phrases: “cancer control”, “cancernetwork”, “structure”, “infrastructure”, “governance”. Moreover, cancer network web sites and cancer control organization reports were consulted using the main following keywords in various combinations: “comprehensive”, “translational”, “cancer”, “network”, “research”, “infrastructures”, “outcomes”.
In order to find literature and examples for care of cancer patients in interdisciplinary networks, a search was conducted a search using the following keywords and phrases in various combinations: “cancer”, “quality of care“, multidisciplinar*, interdisciplinar*, multiprofessio*, interprofessio*, “tumor management group”.
For “Palliative care”, the search strategy for Medline database, which used both text words and MeSH /EMTREE terms, was “palliative care AND network AND cancer AND organization”.
For organizational aspects of cancer research and their link to cancer care outcomes the searched was for the words “comprehensive”, “translational”, “cancer”, “Network”, “research”, “infrastructure”, “outcomes”.
The search was supplemented by reviewing references in the retrieved articles and by searching the authors’ other publications. Because of space restrictions, more recent publications were given priority over older ones, and results were narratively reported to mirror the various dimensions of the issue.
Results
Results from a 2015 survey in which 25 EU Member States participated ( see supplemental information ) indicated that cancer networks do exist in many countries, as institutions share expertise and facilities for cancer services, and that networks can adopt various configurations that may fit the context of individual countries. In some cases, a network evolved from or was built around one or two specialized centres (comprehensive cancer centre or similar) that may coordinate research and services throughout a region or throughout the whole of a smaller country. Elsewhere, nationwide specialized cancer networks coexisted with regional networks encompassing health care institutions (e.g. general hospitals, specialized centres) as well as primary care. The survey moreover suggested that the notion of integrated cancer networks (whether at national or regional level, or whether with a hub & spoke pattern) is gaining ground in response to the needs of contemporary oncology.
In some cases, this notion was expressed in strategic cancer policy documents, whether integrated cancer networks were already at the implementation stage or were yet to be decided upon and formalized. Despite this variety of approaches across regions and countries, networks have in common that they seek to improve and to integrate cancer services, as well as clinical research (8). It emerges from the survey that all the cancer networks included had at least one of the following elements: (i) a formal structure that identifies governing bodies and their respective functions, (ii) multidisciplinary tumour-specific boards that operate according to agreed treatment protocols across the network, and/or (iii) cancer care pathways where named coordinators are responsible for making sure that each patient is seamlessly supported along the pathway at the clinical, psychological and administrative level.
In what follows, the key aspects of CCCNs – their purpose, structure, organization of care delivery and the role of research – will be discussed in more detail, followed by the description of the experiences from a recent CCCN pilot project.
Building a CCCN
Main purpose and decision-making
Inequalities in cancer care exist throughout the cancer continuum. Current evidence suggests that incidence rates and mortality rates from cancer differ across society. There is a higher cancer incidence in the most deprived compared with the least deprived 20% of the population. In addition, for all cancers combined, patients from the most deprived group were more likely to die from their cancer, within five years of diagnosis,) than those from the least deprived group (the difference being as much as 40%; or 27% if adjusted for cancer type (9).
A specific cancer inequality that may be effectively addressed by CCCNs is territorial inequality in access in general and in access to high-quality care in particular, for example represented by inequalities in travel distance to quality cancer care. Territorial inequalities in access to care (i.e. differences in opportunities to receive quality care depending on where you reside, for example due to variable distance from residence to the site of delivery of care or because of local variation of care quality) are a global issue and as such persist with regard to cancer control in the EU Member States.
Often these territorial inequalities conflate in one way or another with other social determinants of health; for example, people of higher socioeconomic status have better opportunities to compensate for longer travelling distances to a higher-quality care centre because they can afford transportation to and from facilities or to pay more to live closer to highquality care. This issue relates to the cancer equity policy paper in that this addresses the issue of inequality in cancer more broadly and to Work Package 7 (community-level cancer care; see Chapter 6), which deals with improvement of cancer care in the community setting (i.e. close to where patients actually live) and the continuation of care after the initial phase of treatment. The current chapter focuses on travel distances to quality cancer care, which is of particular importance since many costs may arise in the follow-up phase (e.g. travelling expenditure) that often patients need to bear themselves.
Recent research shows clear associations between travel distance to a provider and more advanced cancer stage at diagnosis (10,11), guideline adherence, utilization of effective and novel/up-to-date procedures (12–22) and (to be interpreted with particular caution) treatment outcomes (23,24). Some studies however do not show significant results (25,26). There are clear indications that the issue of inequality in access to care particularly affects otherwise disadvantaged patients (15) and, when comparing patients that live in areas with lesser access to cancer care, those with more resources (e.g. indicated by a private health insurance status) tend to receive better care (17).
The organization of cancer services should always aim to ensure that every single patient receives the highest standards of care possible as close as possible to his or her residence. The care of cancer patients should not depend on where they live, where or to which doctor or what level of care (e.g. family doctor, office-based specialist, hospital) they initially present, how familiar they are with existing services or on their socioeconomic level.
A CCCN may achieve these aims by implementing, within its region, integration of activities of several units of primary care and hospital care that provide services to cancer patients, including palliative care and rehabilitation. Also, research conducted in CCCNs may indirectly and directly improve the care of patients. Being multicentred, a CCCN can provide also for patients with limited resources (financial, sociocultural) an access point near home and therapeutic pathways that allow for timely provision of quality services within the network.
Subspecialized services can be provided in one or a small number of locations within a network in order to ensure a critical mass to develop and maintain expertise. Patient transport and continuity of care should be addressed within a CCCN. Achieving these goals is one of the indicators against which the success of CCCNs must be evaluated. The development of therapeutic pathways within the CCCN is of crucial importance to
patients with rare cancers, who may find the different expertise needed for their diagnosis and/or treatment spread among the various units/centres of the CCCN.
Recommendation 5.1
Since physical distance from quality cancer care is a major cause of inequality, it is recommended that clearly defined multiple access points are made available as close as possible to where patients reside, and that uniformly optimal care be provided as close to home as possible.
CCCNs can also be set up also for additional specific reasons depending on the regional and country contexts and the purposes a network is expected to serve.
In the United Kingdom, for example, managed clinical networks were introduced to improve the quality of care and the equality of access (across social or geographic variables), with strengthening of clinical governance, increased specialization of units and more efficient use of resources (2,27,28).
An expert consultation held in 2015–2016 with 11 experts from nine EU Member States (Bulgaria, France, Germany, Hungary, Ireland, Italy, Portugal, Spain and the United Kingdom) revealed that equality of access, quality and safety, as well as continuity of services, were considered key drivers for favouring/ setting up cancer care networks (Fig. 5.2).
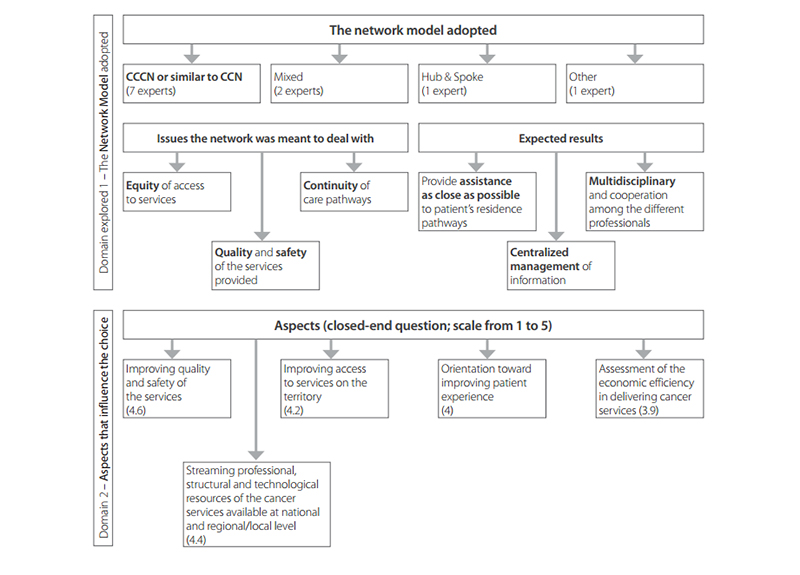
Fig. 5.2 Conceptual map showing the main results of an expert consultation with 11 experts from nine EU Member States (Bulgaria, Germany, France, Hungary, Ireland, Italy, Portugal, Spain and the United Kingdom) in 2015–2016.
Among the most important functions of CCCNs is that of improving outcomes for cancer patients. Current evidence indicates that treatment outcomes for certain types of tumour are likely to be suboptimal in units where very small volumes of activity occur (29,30).
Structure, governance and geographic context
The establishment of a CCCN in a country or region will present new challenges. Because a CCCN may comprise a number of organizations or service units across the patient pathway, it is necessary for the units of the network to work together in a uniform and cohesive manner.
An agreed governance framework is required for the operation of a CCCN that clearly defines the role of each unit/organization participating in the network, as well as for reporting the network’s activities to national or regional authorities. The governance structure of the CCCN will direct all the activities associated with cancer control across the continuum of cancer care. By agreeing on the precise role of each networking unit, the location of specialized services will also be defined. All units will be responsible for setting specific standards for the development and revision of clinical guidelines.
A CCCN board should include representatives of the various stakeholders in the network, including clinicians and patients. The CCCN would also benefit from a dedicated office and executive team, including a director, to provide overall leadership and management for the operation of the network. Appropriate skill mix of staff should include senior managerial and clinical leadership with appropriate administrative support. In practice the suggested governance structure can be adapted to the needs of each network.
In terms of population size, the network will ideally cover an area allowing it to be self-sufficient for the majority of cancer care. Health services should be delivered locally where this is clinically appropriate and possible (31). It is considered that a larger-sized network provides overall patient benefits through central efficiency gains, facilitation of the introduction of service developments and new treatments, increase in the number of patients entered into clinical trials and establishment of internal benchmarking of clinical performance (32).
It is suggested that a CCCN should serve a population of one to two million people (2,33). For very rare cancers, services may have to be centralized to a national or regional centre, into which all local and regional CCCNs feed. In deciding what area should be covered, consideration must be given to geography, administrative status (e.g. regional, supraregional), population size and density for the CCCN, pre-existing cancer services, physical and capital infrastructure as well as workforce capacity. Staffing is a major contributor to both processes and outcomes and is key to the success of a network.
The number of health care professionals and the population they cover can vary widely depending on the country. However, an examination of staffing levels and mix for all oncology professionals (e.g. oncology surgeons, specialist nurses, radiation therapists and other health professionals) in line with international norms is an appropriate starting point (34–38).
There is a strong case for developing a strategic approach to future workforce needs in light of the pace of innovation in cancer care and the increasing demands of an ageing population (29). Integrating workforce planning with service and financial planning can ensure that human resource decisions are linked to the goals of the network.
Recommendation 5.2
We recommend that it be regarded as a priority to establish CCCNs, which are multicentric complexes that bring together units dealing with the management of all aspects of cancer care. These units will be in different locations and under a single governance structure. They will undertake to work together consistently in a structured integrated manner in order to pursue their common goal with greater effectiveness and efficiency.
Outcome indicators, certification and quality assurance
Based on scientific literature and on the testimony of experts regarding possible indicators to be used when planning the network, a set of input, process and outcome parameters has been identified for supporting policy-making and the decision-making process (Table 5.1).
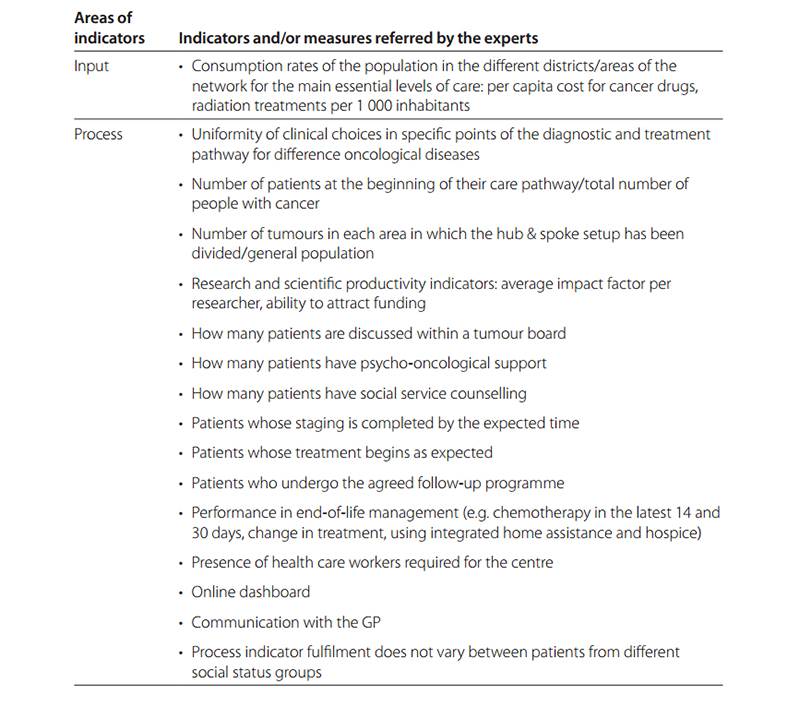
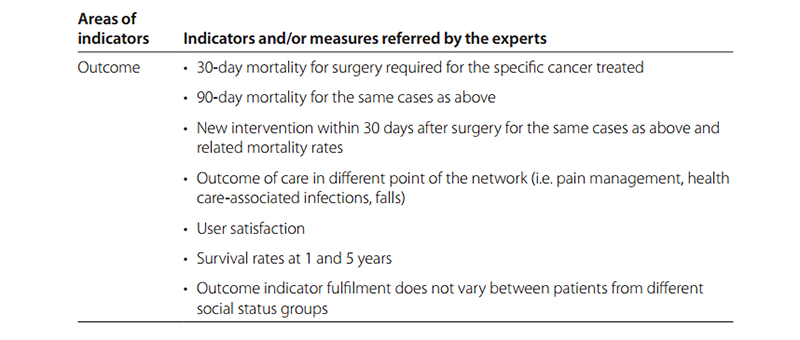
Table 5.1 Possible indicators and/or measures to be used when planning a network
However, this list can be seen as a starting point for future studies that are necessary to appropriately design a set of ad hoc indicators not only for decision-making leading to a CCCN but also to evaluate its implementation.
An important aspect of the functioning of a network is that it continually operates at the highest level of sustained quality and that its operations are of comparable quality to other networks within a country or region. Appropriate governance arrangements to maintain quality in the long term and to provide periodic evaluation to show trends in relevant performance indicators are, therefore, needed.
There are two potential approaches that have been explored internationally. The first is a “national overview panel” that formally assesses the quality of each CCCN. Such oversight is being developed for cancer centres and there are good examples across Europe. In Germany, the German Cancer Society has successfully developed oversight panels that formally assess the quality of all certified cancer centres across the country (39). The alternative is a peer-review process in which individuals from one or more cancer care networks come together to review the work of a separate network drawing on their shared experience of high quality and outlining areas for improvement from their experience, from the evidence-based literature and from national guidelines.
Measuring the performance and quality of cancer care services and programmes is essential to ensure that objectives are being met. Provision of accurate and timely information is a central requirement and this information underpins evidence-based and informed decision-making by policy-makers, researchers, health professionals and patients.
It is necessary to have information technology systems to support improvements in cancer control in the network, with clear legal and administrative frameworks for the collection, sharing and reporting of these data.
Having a unique patient identifier and electronic health record will also facilitate the optimum care of patients across the CCCN. Such data systems will also facilitate the generation of audit and review reports at the network level.
Recommendation 5.3
Given the benefits that a CCCN can provide with respect to equality of access as well as quality of cancer care, it is recommended that the creation of one or more CCCNs is always considered in decision-making. When in a certain area a comprehensive cancer centre already exists, a CCCN can be built with it as its core. Performance indicators and evaluation models should be defined from the outset of the network.
Operation of a CCCN
Care of cancer patients within a CCCN must encompass all phases and all aspects of the disease and aim at all patients receiving continuous, adequate treatment, care and support from appropriately skilled professionals (40). This implies that the network consists of tumour-based multidisciplinary and multiprofessional teams (41). Multiprofessional cooperation in the network contributes to better patient and caregiver satisfaction and improves decision-making and guideline adherence within the network (42–46), although methodological limitations make firm causal associations difficult to establish (47). The teams must include all experts and contact people who are required in order to cover screening as well as all stages of the disease from diagnosis and treatment (involving inpatient and outpatient units), to rehabilitation, follow-up and palliative care.
A defined pathway must be put in place to ensure that patients, regardless of point of access, will be referred to a unit knowledgeable of the disease to start patient management in collaboration with the coordinating referral centre. All steps must be accompanied by psychosocial support (48,49). The use of complementary medicine should be carefully explored with the objective of managing treatment side-effects (50). Special attention must be directed to survivorship of patients, as well as the transition from childhood to adult units for young adults with cancer and the collaboration between paediatric and adult oncology professionals.
Tumour management groups and patient involvement
Tumour management groups are essential to the operation of a CCCN. It is the tumour management group that must discuss a treatment plan for every patient as soon as the diagnosis is made (47), and it is the tumour management group that must periodically review individual steps along the treatment pathway. Each tumour management group must include members of all disciplines and professions required in order to deal with every aspect of the particular type of tumour covered by that group. Each member of the team must be known by name, and the tasks of each must be clearly defined.
Within a CCCN, for at least the most common cancer types, uniform standard operating procedures must be in place; these will be based on tumour-specific national and European guidelines. The equipment for diagnostic imaging, for surgical procedures, for radiotherapy and any other intervention must comply with evidence-based guidelines and must be used according to existing standard operating procedures. The tumour management group is responsible for the development of the standard operating procedures and must critically review if all members of the tumour management group treat the patients according to these procedures and must analyse the quality of care within the network.
All members of a tumour management group must be qualified with appropriate national/international degrees in their respective specialties, and they must update themselves regularly through continuous education. The personal and technical requirements described above should be defined nationally on the basis of the European Guide on Quality Improvement in Comprehensive Cancer Control.
Different treatment options for a specific disease must be discussed with the patients within the context of the participatory decision-making model and patients’ preferences must be taken into account while the patients and the professionals agree on a treatment plan.
Integration
A CCCN is an integrated structure bridging different care sectors such as primary, secondary, tertiary and social care. Bringing links between primary care and cancer services is clearly critically important for timely access to the best treatment. However, integration between cancer services and non-cancer hospital services is equally important in modern practice. Comorbidity in cancer patients requires that their management be shared with specialists in other specialties such as cardiology, respiratory medicine or gastroenterology.
Acute oncology in which patients may be in need of urgent or emergency treatment for the complications of their cancer or complications of cancer treatment (e.g. infection) will usually require close integration of cancer services with critical care services and acute medical and surgical services. Cancer networks cannot, therefore, be developed in separation from general health care services, and the provision of integration with a general health care service is a particular challenge for single specialty cancer hospitals, which are still an important part of cancer services throughout Europe.
Care pathway quality indicators
In order to assess the quality of cancer care within the network, care quality indicators should be identified. Quality indicators should be simple, tumour specific and whenever possible evidencebased and uniform throughout the CCCN. With the implementation of local and national quality assurance programmes (e.g. internal and external audits), quality of care will be visible and, even more importantly, different aspects of care can be observed and compared across providers, for example diagnostics or surgery according to guidelines.
Noticeable results for quality indicators could be discussed within each tumour management group and adequate measures could be defined in order to improve the quality indicator results. Quality indicators can help to implement a plan-do-check-act cycle in the clinical routine. Whenever possible, existing validated quality indicators that are feasible to measure should be used (avoiding developing new quality indicators whenever possible) (45,51–53).
Palliative care
Even if the possibility of better treatment (including target therapies and immunotherapy) has increased life expectancy in many advanced tumours, it has not usually increased the cure rate; therefore, palliative and end-of-life care needs are very relevant for all advanced, progressive and recurrent solid tumours (54,55). Palliative care impact on symptom control and quality of life is evidence based, and more recently the early integration of palliative care in the clinical pathways for patients with advanced disease has also proved to be beneficial for improving quality of life, use of health care resources and, at times, life expectancy (56,57).
Specific improvement in symptom control, firstly pain but also psychosocial and spiritual aspects of care, can be achieved by integration of palliative care in cancer care pathways (58,59). Palliative care delivery requires multidisciplinary dedicated teams and the development of networks of inpatient (hospice and acute hospital), outpatient and home-care services. Specialized palliative care services, at tertiary and general hospitals, hospices, day-hospitals and day care facilities, as well as the local community resources (GPs and home care), are needed to build an integrated palliative care network. Indicators of palliative care integration with oncology (51) are regarded as quality indicators of the clinical pathway; for this reason tumour management groups should include palliative care specialists and palliative care education and research as an important part of the clinical network programme (60,61). Palliative care networks are often available but operate under different organization requirements (62–64); therefore, in most cases it is advisable that the CCCN should work in a structured way with the existing palliative care network.
Rare cancers
Tumour-specific networks are especially important for rare cancers. Rarity is a major obstacle to conducting clinical trials (65) and, therefore, to developing effective treatments and clinical expertise to diagnose and treat these patients (66). Consequently, diagnosis and treatment may lie below optimal standards, particularly when care is delivered by institutions with limited expertise and/or low case volumes. The results of the RARECAREnet survey showed that centres/networks dedicated to rare cancers only exist in a few EU countries, and that centres cover only some of the 198 different rare cancers identified (67). In this context, the RARECAREnet project proposed criteria for the identification of centres of expertise for rare cancers (53). In a CCCN, a defined pathway must be put in place to ensure that patients, regardless of point of access, will be referred to a unit
knowledgeable of the disease to start the patient management in collaboration with the coordinating referral centre.
All this is crucial in order to achieve a timely access to appropriate diagnosis and treatment for patients with rare cancers and to support the concentration of resources and patients essential for translational research. Within a CCCN, one unit should be identified for each rare cancer that meets the requirement stated by RARECAREnet. Among the RARECAREnet-proposed indicators there is the establishment of a quality assurance system to monitoring the services provided. This will be essential to assess CCCNs also on the basis of rare cancer-specific quality indicators. However, in a CCCN, just like in any cancer institute, it is unlikely that there will be the expertise to cover every type of rare cancer. In cases where expertise is not available within the CCCN, the patient should be referred to an appropriate centre outside the CCCN.
Recommendation 5.4
We recommend that a CCCN adopts a multidisciplinary personalized approach based on tumour management groups integrating specialized hospital care with care in the community, palliative care, psychosocial support, rehabilitation and survivorship care plan.
Recommendation 5.5
Quality of care within the CCCN should be measured with quality indicators. A process for continuous quality improvement should be put in place and implemented.
Recommendation 5.6
For each type of rare cancer, we recommend identifying within a CCCN which unit if any can provide the necessary expertise. If for a certain cancer no suitable unit can be identified, the patient should be referred to an appropriate unit outside the CCCN.
Research in CCCNs
There is a growing body of evidence that research-intensive organizations improve the outcomes for their patients as a consequence of their participation in research, and that research-active networks deliver better health care (1–4). Translational and clinical research results in evidence that can have a positive impact on practice, skills and performance and can help to shape the quality of care provided. A CCCN should, therefore, have the capacity to design and conduct integrated cross-disciplinary research programmes and to undertake the dissemination of research results to drive improvement in cancer care.
A CCCN’s closeness to its patient population offers incredible opportunities to advance translational and clinical research, as well as health service and health systems research, and to make new medical breakthroughs, with patients, researchers and care providers working together.
An optimal research organization within a CCCN gathers a critical mass of researchers to support integrated research programmes and has the capacity for successfully translating research results into clinical practice and decision-making. Critical mass equates to the ability to deliver research excellence, with impact and value, and creates further growth.
To facilitate investigator-driven studies across the CCCN, optimal research organization within a CCCN comprises a translational platform that connects patient files and data collection from each centre to facilitate complete longitudinal datasets; common quality-controlled and intercompatible processes for molecular diagnostic and/or other predictive testing; standardized procedures and common standard operating procedures for biopsy procurement and tissue analysis; shared resource facilities (e.g. biological resource, bioinformatics); and linkage to clinical and follow-up data.
All selected examples have developed what may be considered as the core components of an optimal research organization within a CCCN:
- a governance and management structure with executive and scientific committees;
- integrated flagship research programmes, with linkage to clinical practice and professional training;
- shared resource facilities (e.g. biobanks, bioinformatics); and
- dissemination towards and involvement of patient organizations.
Below are selected examples of how research is organized within CCCNs across the world.
France
In France, seven “integrated cancer research sites” (SIRIC) spread across the country have currently been granted this site designation through a competitive international peer-review process to stimulate the pace of cancer research by combining medical services with multidisciplinary research teams. The SIRIC have established appropriate governance structures and developed shared resources/facilities (biobanks with linkage with clinical and genetic data, bioinformatics, etc.). They develop and drive innovative research programmes with strong translational and clinical dimensions and disseminate research outcomes to health professionals, patient organizations and the general public to improve cancer control (68–74).
Germany
In Germany, the National Cancer Plan has defined a three-tier-model of cancer care that comprises organ cancer centres and oncology centres (both certified by the German Cancer Society) and comprehensive cancer centres (or oncology centres of excellence, designated and funded by Deutsche Krebshilfe (German Cancer Aid). The aim of the programme is to support interdisciplinary cancer centres of excellence to set nationwide standards for clinical cancer care and to strengthen translational cancer research. Currently, 13 Deutsche Krebshilfe-designated centres are part of the programme and constitute the German Comprehensive Cancer Centre Network. The goal of this network is to promote innovative developments and set new standards. Elaboration of joint strategies, projects and other activities is accomplished by working groups (75,76).
United Kingdom
In the United Kingdom, there are currently five cancer networks in London. The networks were established with the aim of facilitating seamless care across organizational boundaries (77). The current cancer networks consist of acute trusts, primary care trusts, voluntary sector organizations, and patient and user representatives in the network area, each represented on the network board, which directs and oversees the work of the network. Each network has developed local arrangements to respond to the demands of their population and environment. Networks are expected to link with high-quality cancer research institutions to develop clinical research programmes and enhance their abilities to translate research findings, innovation and education
into improved clinical practice.
Italy
In Italy, regions have achieved various degrees of integration of cancer services (78). The Region of Tuscany, in particular, has organized regional structures into a network that provides oncological services and research activities on cancer. The Istituto Toscano Tumori coordinates the network. “Core research laboratories” in Florence, Siena and Pisa have the objective of conducting innovative research to help to design specific therapeutic strategies for the most effective treatment of these pathologies. Integration of research is taking place not only through the core research laboratories but also through the entire network.
United States
The UC Davis Cancer Care Network is a unique partnership among five cancer centres around northern and central California that includes formal clinical connections to local community cancer centres (which become affiliated to the network through an evaluation process) (79). The UC Davis Cancer Care Network is a specialty-care network linked with an academic health centre. It offers a unique model of care that marries patient-centred treatment with the nationally regarded academic expertise and innovation of a major research university. Comprehensive network services include access to clinical trials, virtual tumour boards, nursing and quality care. The MD Anderson Cancer Network is a collaborative network between the MD Anderson Cancer Center and community hospitals (80). It provides expertise to members from quality assurance and
specialty disease programmes to full clinical integration. Members benefit from the MD Anderson knowledge through access to best practices, leading edge technologies, patient treatment protocols, education, research and a multidisciplinary approach to patient care.
Australia
In Australia, the Hunter Cancer Research Alliance is a multidisciplinary and multi-institutional alliance whose function is to provide capacity-building, funding and strategic support to cancer research across the translational research continuum (81). The Alliance purpose is to enhance collaboration, facilitate training and career development programmes, leverage funding for cancer research projects, develop and maintain essential infrastructure, disseminate research findings and support the implementation of evidence into practice. The Hunter Cancer Research Alliance organizes research efforts across two flagship programmes.
Research clusters in a CCCN
Research clusters in a CCCN may be considered as an essential component of a national cancer strategy, for example for implementing next-generation sequencing platforms and supporting the deployment of experimental pathology platforms and bioinformatics platforms. They are instrumental in structuring new emerging fields, such as cancer immunotherapy, and their coordinated infrastructure attracts industry sponsorship and international collaboration.
Organizing research within a CCCN requires an initial financial investment specifically dedicated to establishing coordination and a management (governance) structure, as well as to help to develop the collaborative platforms and joint resource facilities that are critical to innovative and translational research. A grant-of-designation process conducted by health authorities is critical to the organizational process as it engages all stakeholders and increases shared ownership of the issue of upscaling of cancer care. To optimize CCCN potential, it is recommended that the CCCN scientific committee defines a few high-value flagship research programmes that best fit with their environment.
Recommendation 5.7
We recommend that each CCCN takes full advantage of the proximity of patients, researchers and care providers to pursue basic, translational, clinical, outcome and population research programmes of high quality that will be of high value in supporting the delivery of optimal patient care within the CCCN.