Community-level cancer care, part II
EU policy recommendations for quality improvement in cancer after-care at the community level
This is a part II of chapter Community-level cancer care (Cancon work package 7) of the Guide. See part I.
See the full Guide and other chapters as pdf's.
Cancon Guide is the main delivery of the joint action.
Contents of Community-level cancer care part II
Explorative studies of after-care for cancer patients in five European countries
Discussion
Conclusions and recommendations
References
Community-level cancer care part I
Introduction
Methods
Results
Explorative studies of after-care for cancer patients in five European countries
This section presents five case examples (selection as outlined in the Methods) describing different regional and national approaches to after-care:
• Slovenia: exploration of after-care with their GPs for cancer patients in remission;
• Norway: a standardized, comprehensive patient pathway for cancer patients treated with a non-curative intent but within a clear framework of the cancer strategy;
• the Netherlands: nesting of after-care for cancer patients with GPs and primary care settings;
• Denmark: organization of after-care for cancer patients; and
• Bulgaria: organization of after-care through a network of regional comprehensive cancer centres.
The country information collected serves to give an overview, if limited, of how cancer after-care is organized in European countries and identifies potential advantages and drawbacks of different approaches. This insight will feed into the policy recommendations on how to improve cancer after-care in the future (outlined at the end of this chapter).
The case of Slovenia: experience of GPs with cancer patient after-care
In Slovenia the transition of after-care from the hospital and specialized oncological centres to primary care is still beginning. It was decided to carry out a study that would qualitatively estimate the GPs’ workload with recent cancer patients and then carry out a tentative implementation of a cancer patient pathway for after-care in GP practices/community care. So far, there had been little information about this issue.
The objectives of the study were to describe the existing practices with recent cancer patients in after-care, regardless of their current condition and disease stage; assess the relationships between GPs, other PCPs, social services and families in managing patients with cancer; and provide inputs for a better structuring of the cancer patient pathway in after-care and testing this in a certain number of GP practices.
Survey findings on GPs and GP practice characteristics
Questionnaires were completed in May and June 2015 by 32 GPs (12.8 % response rate): 27 responding GPs were female (84.4%) and the average age of responding GPs was 51.5 years (standard deviation (SD), 7.8).
Data on 160 cancer patients and their treatment were collected via their GPs. More than half (18; 56.3%) of the GP offices were located in a rural area and four (12.5%) were located in nursing homes. The majority of collaborating physicians (23; 71.9%) were employed in a public primary health centre. The community care centre was on the same location as the GPs’ office in two thirds of the cases (21; 65.6%). A single GP had an average of 8501.8 (SD, 3912.5) patient contacts in year 2014, of which 55.6 (SD, 51.5) were house calls. The average number of patients per GP was 1687.8 (SD, 676.2). From those, 99.1 (SD, 95.7) were cancer patients (with active cancer or disease in remission).
On average, 10.5 cancer patients (SD, 10.0) had contacts with their GP in 2014. From all the patients assigned to a single physician, 4.9% (SD, 4.4%) were cancer patients. During 2014, an average of 1.3 (SD, 1.6) cancer patients moved to nursing homes and 6.0 (SD, 5.7) died (all causes of death). Within a period of five working days, GPs had an average of 215.0 (SD, 75.2) patient contacts, from which 13.4 (SD, 11.0) were cancer patients.
Survey findings on cancer patient characteristics and GP satisfaction with hospital and community level care provision
More than half (58.8%) of the cancer patients included in the study were female and their average age was 63.4 years (SD, 14.9). The majority of patients (87.6%) were diagnosed with cancer after 2005. For patients included in the study, time to diagnosis was an average of 5.7 weeks (SD, 4.8). The most common diagnosis was malignant neoplasm of breast, affecting nearly a quarter of the cancer patients in the study (23.4%). Among the common diagnoses were malignant neoplasms of digestive organs (22.1%), malignant neoplasms of male genital organs (9.7%), malignant neoplasms of female genital organs (8.4%) and melanoma and other malignant neoplasms of skin (8.4%).
Patients contacted their GP on average 10 times (SD, 9.6) in the year before the study. The majority of visits (6.2; SD, 6.6) were of administrative nature and consisted of issues such as issuing prescriptions, referrals and medical device ordinances. Less frequently, the purpose of the visits was for coordination of health care services (2.0; SD, 3.6), consultation with relatives (1.9; SD, 6.0) and psychosocial support (1.6; SD, 3.0). Coordination of social services, help with activities of daily living, palliative care and home care were seldom topics of GP visits. Disability evaluation was not undertaken frequently because the majority of the patients were no longer in active employment. GPs were mostly satisfied (5.6 to 4.1 on a 7-point Likert scale) with patient treatment at primary and secondary care levels, existing guidelines, diagnostics possibilities, accessibility of services at the secondary care level, accessibility of pharmacological pain management and with information transfer from specialists to GP.
They were somewhat dissatisfied (from 3.1 to 2.1) with medical oncall services, inclusion of family members in treatment process and with communication with specialists involved in cancer patient treatment. They were mostly dissatisfied with palliative care, willingness of other services to be involved in cancer patient treatment, accessibility of non- pharmacological management of pain and other symptoms, organized home care and with community care services. GPs were most dissatisfied (from 1.9 to 0.4) with availability, involvement and coordination of community and social care services. However, patients seldom used these services.
Interview results
Findings from the interviews in May and June 2015 showed that physicians experience high levels of stress particularly in the early phases of cancer patient care – from time suspicion of cancer is established to time of cancer therapy initiation. In order to shorten the time before the patient with a suspicion of cancer receives diagnostic tests, GPs have to coordinate health care services. Physicians reported lack of “fast track” for suspected cancer patients, particularly in cases based on an agreement between GP and specialist. They would also value a possibility of consultation with a specialist in cases of suspicion of cancer where future management is unclear. Patients rarely meet their GP while being treated at the Institute of Oncology, which scored high in satisfaction with the treatment delivered as perceived by the GPs. While cancer patients are treated in ambulatory settings, more contacts are needed with their family members than with the patients themselves.
GPs emphasized the importance of cooperation with different health care service (community care, patient transport services) during the ambulatory cancer treatment phase. GPs did not report special needs or problems during the surveillance phase of cancer care. However, they did report that there should be more emphasis on full rehabilitation of the patient, including psychosocial support. Furthermore, the interviewed physicians reported that patients need more psychological support during the after-care period. Easier access/referral to psychotherapy would benefit patients, particularly in reducing fear of cancer recurrence, according to the GPs. Respondents reported that they had struggled with palliative care of patients in the past because of lack of knowledge but the palliative care area had improved vastly in recent years. They reported having numerous palliative care courses and a specialist of palliative care available for consultation. The latter is an exception occurring in the region of Slovenia from which the interviewed physicians originate (Upper Carniola).
Development and testing recommendations
An observational retrospective study included 13 out of 32 GPs (40.6% response rate), who had already participated in the first round of the study. Data on 63 cancer patients and their treatments were collected and evaluated. Activities, most frequently performed (over 70%) included general and psychological support as well as good communication skills and care for concomitant chronic diseases. Elaboration of a written treatment and pain management plan, coordination with other community services and psychosocial rehabilitation plan were less frequent activities (under 15%). In general, there was less need for assessment of occupational disability (in 23% of patients), because most patients were already retired. Among those of working age, more than half were involved in activities related to occupational assessment and rehabilitation. GPs evaluated good practice recommendations as a useful, although sometimes too general, tool in cancer after-care; however they emphasized time shortages in comprehensive after-care. Suggestions were raised that nurses could also take part in cancer after-care.
In summary, GPs in Slovenia have an increasingly important role in the after-care of surviving cancer patients with the majority of contacts occurring for administrative reasons. However, GPs experience high burden of stress during the initial phases of a patient’s disease as well as later during after-care. Cancer patient pathway (i.e. good practice recommendations) was recognized as valuable tool for systematic approach to patients with cancer during after-care.
TopThe Norwegian experience: the development of an integrated care pathway (the Orkdal model)
Norway decided in 2013 to strengthen the coordination of care at the regional level and introduce integrated care pathways (ICP) as a method to implement this integration into clinical practice; ICPs are structured multidisciplinary care plans that can facilitate a process by which palliative care and oncology can be integrated in a given setting. ICPs may provide a process plan, provide a time frame, describe the type of expertise needed at any step in the process and describe the resources needed during the trajectory (13,14). A recent Cochrane review concluded that ICPs reduce hospital complexity and improve documentation without having any negative impact on length of stay or hospital cost (15).
The ICP
The main component of the Orkdal model is an ICP that facilitates evidence-based practice, improves coordination of care in all phases of the disease trajectory, and integrates oncology and palliative care. The care pathway is to be applied regardless of cancer diagnosis, focusing on function, needs and symptoms, and it covers health care services in home care, nursing homes and specialist care. Symptom assessment and optimal symptom management, definition of responsibilities, optimal communication and access to cancer care services whenever needed constitute the core of the pathway. Overall, the development and use of the ICPs contributes to ensure:
- quality of palliative care offered (equal quality of care regardless of level);
- responsibility in that there is a clear definition of which health care professional is responsible for the patient at different points in time (defining the right level of care);
- flexibility as the patient’s needs vary during the disease trajectory and may often be difficult to predict; and
- availability of health care services 24 hours a day/seven days a week in order to ensure safety and quality for patients and carers.
The Norwegian model for comprehensive cancer care is the basis of the ICP where GPs (with a gate-keeping role) and home-care nurses are responsible for the visiting, treating and caring of the patient at home and in community care settings (16). Specialists are available for the GPs for supervision. Patients’ needs are considered in a step-up model: the first option considered is the local nursing home with specialized oncology/palliative care, then local hospital care and finally, for highly selected patients, university hospital care (Fig. 6.6). Necessary templates, checklists, assessment tools, contact information and relevant guidelines are included in the ICP. Electronic assessment of patient-reported outcomes is partially applied in the project.
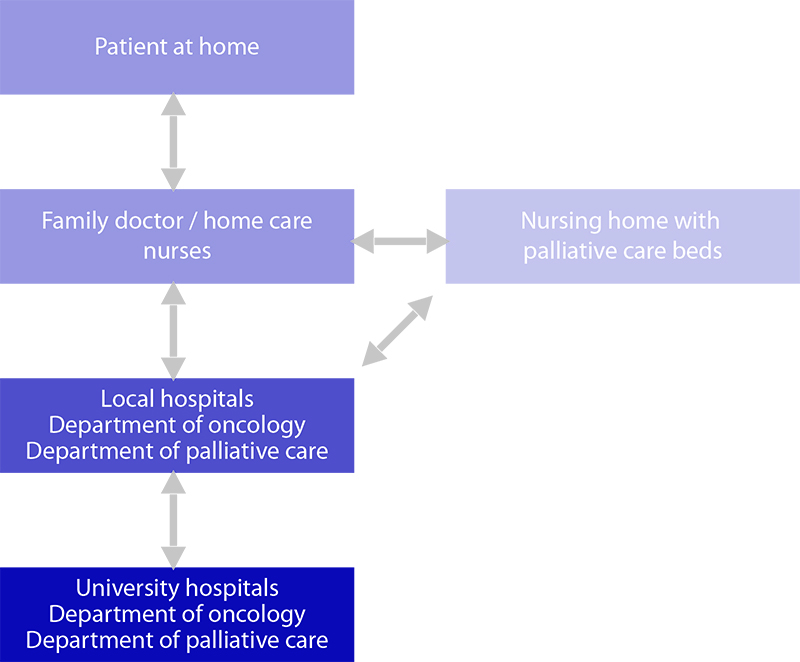
Fig. 6.6 Norwegian comprehensive cancer care
Educational programme
To implement the ICP, an educational programme in oncology and palliative care is offered to the providers, mainly to physicians, nurses, and nurse assistants. Participants are encouraged to teach colleagues at their respective place of work. Resource cancer nurses organized in a network have extra responsibility for teaching and for implementing the model locally. Project positions for GPs and community cancer nurses have been offered at the outpatient clinics to achieve specialist competence in community care. A master’s programme in pain and palliative care has been established at the Norwegian University of Science and Technology, Trondheim.
Information for citizens
Through the project, patients, carers and the public are offered information regarding chemoand radiotherapy, symptom diagnosis and treatment, available expertise, volunteers, educational courses for patients and carers, and who to contact. The information is given as written materials, electronically and at public meetings. Educating patients in systematically reporting of needs and symptoms and about available oncology and palliative services is important to achieve highquality care where the patient is actively taking part in treatment decisions and planning of their care.
Advantages of the integrated cancer care pathway
The care pathway increases the quality of care (17). Some of the experienced advantages (17–20) that have been identified so far in the participating municipalities are:
- systematic symptom assessment and evaluation of different treatments according to the patient reported symptoms, function and needs;
- higher degree of collaboration and better coordination of services, including fewer unnecessary consultations and better planning of what services the individual patient and family may need;
- clarification of treatment intention and a clear treatment plan; fewer unnecessary potential toxic anticancer treatments;
- medication lists updated more often;
- improved communication between health care providers across levels of care, including better written reports that are available at the time of change of place of care;
- improved communication between health care providers and the patient;
- improved communication between health care providers and the carers; and
- support from management in the specialist and community care needed to succeed with implementation.
Advantages of the educational programme
After participating at courses focusing on integrated cancer care, the GPs are more aware of the cancer patients and their potential needs for closer follow-up in all phases of their disease (i.e. after being cured, living with metastatic disease and at the end of life). PCPs in community care are more aware of further available expertise locally and in specialist care. Because they have support from specialist care, they are to a larger extent able to take more responsibility locally and for patients with complex cancer. GPs and cancer nurses educated thorough project positions at the outpatient clinic now working locally and are providing specialist competence at community care level, which is made possible through more health care providers locally having more knowledge in oncology and palliative care.
Consequently, oncologists are able to spend their time specifically on oncological treatment and the most complex disorders. Furthermore, fewer hospitalizations may be needed and potential toxic anticancer treatment might be stopped earlier or largely not started when appropriate.
Advantages of information for citizens
Better-informed patients and families make in many cases better treatment decisions and improve communication. The patients may feel more confident and that the disease trajectory is more predictable. Patients may report symptoms more reliable and may be better educated to ask for available services such as appropriate treatment for pain and nausea or advice from a social worker. In summary, the ICP, educational programmes for PCPs and information delivered to citizens increase quality of care, improve communication across levels of care and with patients and contribute to better treatment decisions.
The Netherlands: the role of primary care in after-care for cancer
In 2013 and 2014, NIVEL published a series of papers based on studies that analysed the characteristics of cancer patients in primary care, the resulting workload and the consequent impact on the development of these services at the primary care level in the Netherlands. Key findings of these studies are summarized below.
Primary care for cancer survivors
While Dutch GPs have no formal role in follow-up visits in the first five years after diagnosis of cancer, they are involved in care for cancer survivors. As many cancer patients are older and have chronic diseases in addition to cancer, they visit their GP for various other health problems. At the end of the follow-up visits with the medical specialist, GPs take over the care for patients.
The number of cancer survivors per GP practice is considerable. In an average practice of 2350 listed patients (1.0 full-time equivalent GP), about 70 patients had been diagnosed with cancer less than nine years previously (21). Cancer survivors have more GP contacts than those of the same age and sex without cancer (Table 6.7). In the first years after diagnosis, cancer survivors have a higher number of office visits and telephone consultations and the GP more often pays home visits.
Cancer survivors also have a higher number of medication prescriptions and referrals to secondary care (22–25). Patients who are over 60 years of age and have been treated with breast-conserving surgery may be referred to their GP for yearly follow-up visits, with a mammography every two years. After the initial treatment for prostate cancer, clinical examination and PSA measurement are recommended after six weeks, at three, six and 12 months, and then every six months for the three years after diagnosis and annually from five to 10 years. After five years, patients with stable low PSA levels may be referred to the GP.
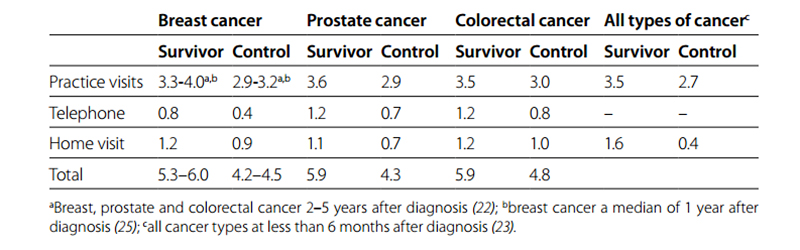
Table 6.7 Mean number of GP contacts per year in Dutch cancer survivors compared with age- and sex-matched controls without cancer from the same GP practice
The number of GP contacts varies widely between patients. As expected, older patients and those with a chronic disease have the highest number of GP contacts. Cancer survivors aged 50 years without a chronic disease have, on average, three to four GP contacts per year, while those aged 80 with a chronic disease have, on average, eight to nine contacts per year. The increase in the number of GP contacts with age and with the number of chronic diseases is similar in cancer survivors and controls without cancer (26). Health problems for which cancer survivors visit their GP The health problems for which cancer survivors visit their GP differ by cancer type. Breast cancer survivors visit their GP more often for acute symptoms, such as back or abdominal pain, in the period of two to five years after diagnosis. They also visit the GP more frequently for common infections, such as cystitis or respiratory infections (27). The GP also frequently take over the management of hormone or aromatase inhibitor use, which was originally initiated by a specialist. Although breast cancer survivors do not visit their GP more often for psychosocial problems, GPs more often prescribe psychomedication to these patients. In the two to five years after diagnosis, prostate cancer survivors visit their GP more often for general symptoms, such as fatigue, constipation and back pain, but surprisingly not for urinary incontinence or erectile dysfunction. Colorectal cancer survivors visit their GP more often for infections, such as skin or urinary infections, but also because of anaemia, abdominal pain and side-effects of treatment.
In conclusion, cancer survivors visit their GP more often for common acute symptoms, such as fatigue, pain and common infections. This may be related to late effects of cancer treatment. Both fatigue and pain often develop during treatment and may persist thereafter. Infections may occur because of a weakened immune system. Alternatively, cancer survivors may be more prone to visit their GP for these relatively common symptoms because of increased health concerns. The number of GP contacts related to chronic diseases and psychosocial problems is slightly higher in cancer survivors but is not a major cause for the increase in health care use. Estimated increase of GP contacts in the Netherlands in the future Following the increasing incidence of cancer and improving survival for cancer, it is expected that the number of patients living with cancer in the Netherlands will increase from 419 000 in 2009 to 666 000 in 2020 (28). As these patients frequently contact their GP, this will also lead to an increase in GP contacts. Researchers from NIVEL have estimated the rise in contacts will follow two scenarios. The first scenario takes into account the estimated increase in the number of cancer survivors. The second scenario also takes into account the current debate to increase the role of the GP for after-care. GPs are already involved in care for these patients; they often see these patients for chronic diseases besides cancer and their practices are usually near to their patients’ residences. It is, therefore, suggested that part of after-care should be transferred from the specialist to the GP. In the second scenario it is assumed that this will lead to two additional GP contacts per year.
In Table 6.8, the estimated number of contacts in the Netherlands and the number of contacts for a standard GP practice (with 2350 listed patients) is given (29). It is estimated that in 2020 a standard GP practice will have 850–1100 contacts with cancer survivors, about 20 contacts per week. This is an increase of 70–120% compared with 2010.
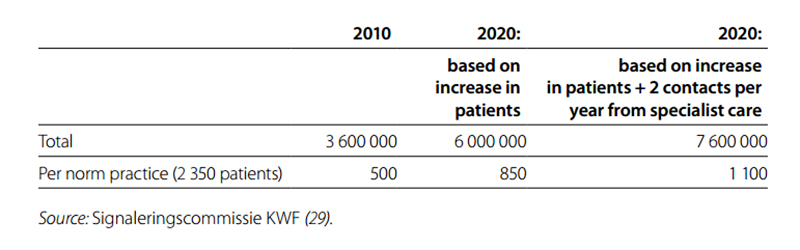
Table 6.8 Number of contacts per year that GPs have with cancer survivors in the Netherlands
In summary, GPs in the Netherlands have an important role in the after-care of surviving cancer patients, as demonstrated by the higher number of contacts compared with patients without cancer. Cancer patients visit their GP for common health problems. With the increasing number of surviving cancer patients, GPs might be also involved in the future in oncological after-care for surviving cancer patients. Denmark: organization of cancer after-care in primary care settings In Denmark, a reform of after-care is in progress, following reports of waste caused by unnecessary oncological hospital-based follow-up of cancer survivors. This reform also comes out of need for rational use of the resources in cancer care before an expected increase in cancer patients caused by the ageing population. The main characteristics of this ongoing reform process are described below.
The working process and timeline Following discussions in 2010–2012 on the need for a reform in cancer after-care, the decision to reform was taken in 2012. In 2013, a specified working plan was agreed where the Danish Health Authority would chair a series of working groups that would describe the follow-up for each main type of cancer.
These plans on follow-up care would be worked out in collaboration with relevant administrative and clinical stakeholders (the multidisciplinary national cancer groups) including representatives from general practice. The plans would be amendment to the already created national fast track system for diagnosis and treatment of each cancer. The implementation of the reform was scheduled for the end of 2015 and the beginning of 2016. The content of each plan Each plan would follow a standardized scheme and it was printed (some 30–40 pages) and put on the web by the National Board of Health (30).
The following items would be addressed in each plan:
- an individual needs assessment for each patient and clear agreement with each patient about the follow-up plan following completed primary treatment;
- description of symptoms and signs of recurrence and strategy to identify recurrence;
- rehabilitation and palliation assessment and planning;
- psychosocial, spiritual and existential considerations including ways to empower patients and involve them; and
- the evidence base for the recommendations in each guideline would be identified.
Implementation in 2016
Fourteen groups have developed 19 new guidelines with eight in the implementation phase. The five regions, which are also the hospital owners, have a strong focus on the implementation process, which they try to follow in a nationally agreed form. There are still not confirmed agreements on information technology monitoring and monitoring of the process. Each plan has a description of some sort of a stratified approach with tasks for specialists, nurses in hospital and for GPs.
The implementation will follow three steps.
- Breast, gynaecological cancers, colorectal cancer and prostate cancer (under implementation)
- Head and neck, brain, sarcoma, melanoma
- The rest of cancers.
There is still no agreed work plan and payment plan for GPs involvement, but it is anticipated that they only will be involved to a larger degree in prostate cancer and for the other cancers; their role will probably be patient attendance on an ad hoc need-based way. There is no specific agreement for how to establish an integrated trajectory as patients will be (for now) regarded as oncological patients and not transferred/referred to primary care. There is no specific agreement on how GPs may access specialized services, including fast and direct investigation methods and help from consultants.
Bulgaria: organization of cancer after-care in regional cancer centres
Follow-up of patients within the Bulgarian health care system is part of the development and implementation of activities and services within the framework of a more comprehensive approach to cancer concentrated within specialists’ expertise in comprehensive cancer centres, not within primary care. In this setting, how do comprehensive cancer centres deliver after-care and are patients and professionals satisfied with this arrangement?
Seven comprehensive cancer centres were approached and four of them returned completed questionnaires: Sofia, Veliko Tyrnovo, Burgas and Stara Zagora (detailed information from the questionaires is provided as supplemental information at www.cancercontrol.eu). The results indicate the small number of doctors working within a comprehensive cancer centre in comparison with the very high numbers of patients and examinations conducted. Despite the fact that consultation with relatives is part of a clinician’s practice, provision of psychosocial care within the centres is usually lacking. In relation to coordination of health services and social services, comprehensive cancer centres are not reported to have such responsibilities.
Comparison of data on doctors’ and patients’ experiences indicates several significant differences. There is a gap between the satisfaction expressed by doctors and that by patients. When asked about their experience of treatment of oncological patients at the secondary level, patients were largely satisfied, while the doctors were dissatisfied. The same discrepancy emerged in terms of participation of other services in the treatment of patients. Organized home care (when needed) was considered unsatisfactory by the doctors, while for patients it was satisfactory. Doctors felt that the involvement of family members in the care of the patient and the role of the community social care services were areas that would need further improvement (overall reported as being neither satisfied, nor dissatisfied) whereas these two topics were reported as being satisfactory in terms of patients’ experience.
When comparing results from doctors and cancer patients, it was established that there are some clear similarities, such as satisfaction on existing guidelines, availability of pharmacological substances for pain relief, communication of clinical specialists in after-care and replacement in case of absence. The specific characteristics of the health care system and the role of GPs in oncological treatment and after-care were evident in the level of dissatisfaction about issues of treatment of oncological patients at the primary level experienced both by the doctors and by the patients.
The qualitative interviews showed that GPs consider the provided after-care for cancer patients as being inadequate and the patients’ needs unmet (in terms of psychological and social support and the feeling of isolation in the process of treatment). In terms of patients’ experience, there is a strong assumption about feelings of dissatisfaction as usually the patient has to wait for treatment and after-care because of the large number of cancer patients and the limited number of medical specialists; consequently, this situation causes more distress. Moreover, it is a huge obstacle for the patients to remain in employment and to keep up the “unnecessary” bureaucratic procedure related to it.
The prospect of engaging the GPs within the process of after-care is not considered a possibility. In reality, doctors’ expectations of primary care are regular monitoring and home care of cancer patients, assistance in diagnosis and better electronic communication. Doctors feel that within the primary levels, GPs have to provide services such as examinations, psychosocial care, home care, help with the activities of daily living, prescriptions, planning of care and working disability assessment. Other experts and organization should assist in issues of palliative care, coordination of health and social services and family consultations.
TopDiscussion
The changing developments in cancer diagnosis result in a growing number of people who survive cancer (31). As a result, the role of after-care is becoming increasingly important, but also challenging from the point of view of the volume of this care. Oncologists in hospitals often provide cancer after-care (32) but are facing challenges, such as limited hospital capacity and increasing patient numbers. At the health policy level, there are pressures to substitute care by replacing specialist-led care by GP-led care. Consequently, there are increasing calls for greater involvement of GPs in the after-care of cancer survivors. To give PCPs a greater role, however, raises the question of what information and resources GPs can draw on to provide after-care.
This chapter give an overview of evidence- and opinion-based recommendations on after-care for cancer survivors that are (potentially) relevant for GPs. Guidelines on the five most common tumour types were studied and information on three categories – recurrence detection, long-term effects and recurrence prevention – was provided for all tumour types. The inventory highlights that there is not always sufficient evidence on the best way to provide after-care nor conclusive proof about the optimal frequency of after-care diagnostic testing. Furthermore, most information provided by guidelines was not evidence based, indicating a need for research on after-care for cancer survivors. There is a clear need to improve guidelines with respect to the different providers who are increasingly involved in after-care as well as training of GPs and other PCPs, because they will face rising patient needs that will realistically be covered only by the PCPs.
The chapter also presents an overview of national and regional practices on how cancer after-care is organized in Bulgaria, Denmark, the Netherlands, Norway and Slovenia. Four of these countries have a GP-gate-keeping system and a strong role of community care, including in the cancer aftercare process. Only in Bulgaria does after-care remain the task of cancer hospitals.
In Slovenia, GPs coordinate health care services during the early diagnostic period of cancer patient care. Later, patients rarely meet their GP while being treated at the specialist centres. In after-care, the majority of clinical visits are of administrative nature, but they also include coordination of health care services, consultation with relatives and psychosocial support. GPs emphasized the importance of cooperation with different health care services during all phases of cancer care.
Norwegian experience is based on ICPs that facilitates evidence-based practice, coordination of care and integration between oncology and palliative care. Use of ICPs contributes to quality of care, clear definition of responsibilities, a flexible approach to patient’s needs and availability of services. The care pathway involves home care, nursing homes and specialist care.
Dutch GPs are involved in the care for cancer survivors not earlier than five years after diagnosis of cancer. They provide consultations for common problems or late effects of cancer treatment. Cancer survivors have more GP contacts than patients without cancer.
In Denmark, a reform of after-care is in progress. It is expected that plans on follow-up care will be prepared for each main type of cancer. Involvement of GPs in planned after-care is not yet agreed and GPs will probably see patients on an ad hoc needs-based way.
After-care in Bulgaria is provided in comprehensive cancer centres. Coordination with home and social care services is often poor, although patients feel satisfied with it. Stronger involvement of GPs is not considered as a relevant option.
The five different country cases have only few elements of cancer after-care in common. Countries with GP-based system implemented comprehensive (e.g. Norway, Denmark) or fragmented (e.g. Slovenia) mechanisms of coordination across levels of care. These mechanisms range from a full integration model (e.g. Orkdal model, Danish reform), where care pathways link providers, professionals and services around patient needs, to a fragmented approach, where coordination of care is a matter of GP’s core competencies rather than systematic (e.g. Slovenia). Dutch experience fits between these two. Bulgaria, as an example of a country with centralized cancer care, struggles with scarce resources and after-care is not at the top of the agenda.
All these challenges are important, as is the need for sharing all patient information and developing joint files. Seamless care requires proper access to all data and information through linkages between clinical data and outpatient and primary care data and registries. Such an approach would be helpful in providing continuity and comprehensive information for everyone involved in continued cancer care.
TopConclusions and recommendations
Europe presents a wide variety of approaches in the organization of cancer after-care. In many cases, hospitals and oncological services at the in- and outpatient level continue to provide a large part of after-care. Under such circumstances, the role of PCPs remains supportive to the process and important in terms of securing all the other services and for integration with other sectors to cover issues such as patients’ employment and material and psychological needs. PCPs also need to ensure proper care for other chronic conditions that these patients may have at the time of the cancer diagnosis or that may be diagnosed later. Such patients will inevitably become frequent users of community care services.
Nevertheless, as cancer incidence continues to increase, and modern oncological care becomes ever more specialized, focused and intense, the question arises as to how high-quality after-care and long-term supervision for stable patients in remission can be organized while taking their diverse needs and expectations, as well as cost-effectiveness, into account. Cancer is a specific noncommunicable disease that can be successfully treated and cured, unlike most of the other noncommunicable diseases. This means that overcoming cancer is a reality and leads (former) patients, their carers and the professional staff treating and monitoring them into new challenges related to survivorship.
In this exploration of cancer after-care services and the typology of providers, the experiences of five different countries were examined, some of which were going through a transformation of their existing practices. The selection of countries depended on the willingness of their respective ministries of health and the representative institutions to participate, and not on any predefined set of criteria. Nevertheless, we believe that this insight into after-care practices provides valuable insight into the current state of after-care as well as for the potential for improvements.
Our objective was to propose a blueprint for a cancer patient pathway in after-care. After the survey on after-care in EU Member States, and also based on some previous experience in the participating countries, it became clear that such a pathway would not be feasible. Health care systems, the modalities and mechanisms of their financing and the traditions across countries vary importantly and do not allow for a higher level of uniformity. In spite of this conclusion, we believe that the contributions from the participating countries are important for the following reasons.
- We have been able to obtain clear and comprehensive data on the existence and applicability of guidelines for after-care and discovered both discrepancies in information and guidance as well as in the provision of after-care.
- We could identify the need to dedicate more time to the development of prevention guidelines for cancer survivors, focusing particularly on secondary and tertiary prevention, while not forgetting about health determinants.
- We have explored five countries with different approaches to after-care and which are at different stages in the transition from hospital to primary care:
- Bulgaria, where provision of after-care is limited to cancer centres, which is a model that might work when both patient expectations and PCP’s wishes are supporting such a choice;
- the Netherlands, where important information on long-term challenges for PCP posed by cancer survivors are highlighted, including increased workloads, spousal and carer burden and additional training and knowledge needs;
- Denmark, where a transition process, ongoing in 2016, is intended to integrate patient aftercare among specialists, nurses in hospitals and GPs;
- Norway, where a country with geographical challenges is trying to find ways to bring survivorship support, after-care and palliative care closer to where patients live (without losing the quality and efficiency of these types of care); and
- Slovenia, where GP practices appear to be overwhelmed with the challenges presented by increasing numbers of with cancer patients but are developing elements of a future care pathway for after-care for cancer patients.
Recommendations
This overview gives policy-makers/guideline developers the opportunity to discuss different after-care topic actions, tests and awareness, sometimes supplied with frequencies that could be included into their own guidelines on after-care for a specific tumour type.
It also shows that preparing a comprehensive/integrated patient pathway is important for several reasons.
- seamless care is needed and expected; the care needs to be continued across the formal institutional boundaries;
- patient information is crucial; patients need to be fully and comprehensively informed about the processes related to their continued care; and
- guideline implementation is needed (when and where these are in place) to structure care around the evidence-based milestones (patient pathway represents a common tool for guideline implementation).
For the future development of cancer after-care we recommend the following.
- 1 Manage cancer as a continuous process where patients seamlessly pass (transit) different phases and stages. This can be achieved through the creation and updating of a cancer patient pathway going from screening outcomes through diagnostics and treatment to long-term monitoring in remission, life-prolonging treatments and palliative and end-of-life care. It is important to:
a/ reflect the current level of knowledge in cancer treatment but also the specifics of the country’s health care system and its organization;
b/ secure the necessary resources, human, financial, equipment and medicines, at all stages of the pathway;
c/ develop the segment of the pathway for the cancer patients’ after-care in close collaboration between specialized oncological care and PCPs; and
d/ organize an information exchange platform that enables all providers involved in cancer patient care to share the data and files relevant to the patient. - Organize the education and training for PCPs in order to strengthen their capacity to cope with the increasing population of cancer patients in after-care.
- Develop guidelines and guidance, at least for each of the most frequent cancers, on what to include and on what not to include in the long-term monitoring of patients (system specific, differences in access to some tests and diagnostics). This should include the following segments:
a/ recurrence detection, indicating the best frequency to perform diagnostic tests to detect cancer recurrence, the description of the signs and risk of recurrence in a given category of patients and, finally, defining and elaborating for the patients’ after-care in terms of the responsibilities of GPs (in case they are willing to perform this role);
b/ long-term effects of cancer, where there should be more information on the potential complications of individual types and locations of cancer and how these should be prevented and treated; furthermore, more knowledge and recommendations on psychological support for cancer survivors are warranted; and
c/ recurrence prevention, where there should be more research into the value of recurrence prevention and specific recommendations for cancer survivors. - Coordinate services between the health and other sectors for many patients not only for those who become disabled or are terminally ill. Treatment itself, long absences from work or treatment away from family may raise all sorts of problems (e.g. additional expenses or less productivity).
References
1 Grunfeld E, Earle CC. the interface between primary and oncology specialty care: treatment through survivorship. Journal of the National Cancer Institute Monographs, 2010;40:25–30.
2 Wood ML, McWilliam CL. Cancer in remission: challenge in collaboration for GPs and oncologists. Canadian GP, 1996;42:899–910.
3 Hoekstra BA, Heins MJ, Korevaar JC. Health care needs of cancer survivors in general practice: a systematic review. BMC Family Practice, 2014;15:94.
4 US Agency for Healthcare Research and Quality. AHRQ’s national guideline clearinghouse is a public resource for summaries of evidence-based clinical practice guidelines [web site]. Rockville, MD, US Agency for Healthcare Research and Quality; 2017 (www.guideline.gov, accessed 14 January 2017).
5 Guidelines International Network [website]. Pitlochry, Scotland, Guidelines International Network; 2016 (www.g-i-n.net, accessed 14 January 2017).
6 Jatoi I et al. Hazard rates of recurrence following diagnostic tests of primary breast cancer. Breast Cancer Research and Treatment 2005;89:173–178.
7 Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database of Systematic Reviews, 2007;(1):CD002200.
8 Kobayashi H et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery, 2007;141(1):67–75.
9 Demicheli R et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. Journal of Thoracic Oncology, 2012;7:723–730.
10 Francken AB et al. Follow-up schedules after treatment for malignant melanoma. British Journal of Surgery, 2008;95:1401–1407.
11 Meyers MO et al. Method of detection of initial recurrence of stage II/III cutaneous melanoma: analysis of utility of follow-up staging. Annals of Surgical Oncology, 2009;16:941–947.
12 Turner RM et al. Optimizing the frequency of follow-up visits for patients treated for localized primary cutaneous melanoma. Journal of Clinical Oncology, 2011;29:4641–4646.
13 Norwegian National Ministry of Health and Care Services. Together – against cancer. National Cancer Strategy 2013. Oslo, National Ministry of Health and Care Services; 2013.
14 Ferlay J et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European Journal of Cancer, 2013;49(6):1374–403.
15 Rotter T et al. The effects of clinical pathways on professional practice, patient outcomes, length of stay, and hospital costs: Cochrane systematic review and meta-analysis. Evaluation of Health Professionals, 2012;35(1):3–27.
16 Ministry of Health and Care Services. The coordination reform – proper treatment – at the right place and right time. Oslo, Ministry of Health and Care Services; 2009 (Report No. 47 to the Storting; Norwegian; https://www.regjeringen.no/en/dokumenter/report.no.-47-to-thestorting-2008-2009/id567201/, accessed 14 January 2017).
17 Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. Journal of Palliative Medicine, 2000;3(3):287–300.
18 Hunt KJ, Shlomo N, Addington-Hall J. End-of-life care and achieving preferences for place of death in England: results of a population-based survey using the VOICES-SF questionnaire. Palliative Medicine, 2014;28(5):412–421.
19 Miccinesi G et al. End-of-life preferences in advanced cancer patients willing to discuss issues surrounding their terminal condition. European Journal of Cancer Care, 2012;21(5):623–633.
20 Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for palliasjon i kreftomsorgen [Palliative care in cancer care: action]. Oslo, Helsedirektoratet; 2013 (Norwegian; http:// helsedirektoratet.no/publikasjoner/nasjonalt-handlingsprogram-med-retningslinjer-for-palliasjoni-kreftomsorgen-/Publikasjoner/nasjonalt-handlingsprogram-for-palliasjon-i-kreftomsorgen.pdf, accessed 14 January 2017).
21 Korevaar JC et al. Oncologie in de huisartspraktijk. Huisarts & Wetenschap, 2013;56:6–10.
22 Heins M et al. Determinants of increased primary health care use in cancer survivors. Journal of Clinical Oncology, 2012;30(33):4155–4160.
23 Jabaaij L, van den Akker M, Schellevis FG. Excess of health care use in general practice and of comorbid chronic conditions in cancer patients compared to controls. BMC Family Practice, 2012;13:60.
24 Brandenbarg D et al. Increased primary health care use in the first year after colorectal cancer diagnosis. Scandinavian Journal of Primary Health Care, 2014;32(2):55–61.
25 Roorda C et al. Increased primary healthcare utilisation among women with a history of breast cancer. Support Care Cancer, 2013;21(4):941–949.
26 Heins MJ et al. The combined effect of cancer and chronic diseases on general practitioner consultation rates. Cancer Epidemiology, 2015;39(1):109–114.
27 Heins MJ et al. For which health problems do cancer survivors visit their general practitioner? European Journal of Cancer, 2013;49(1):211–218.
28 Signaleringscommissie KWF. Kankerbestrijding. Kanker in Nederland in 2020 [Cancer in the Netherlands in 2020]. The Hague, Signaleringscommissie KWF [Signalling Commission for Cancer of the Dutch Cancer Society]; 2011.
29 Signaleringscommissie KWF. Nazorg bij kanker: rol van de eerste lijn [Follow up for cancer: role of primary care]. The Hague, Signaleringscommissie KWF [Signalling Commission for Cancer of the Dutch Cancer Society]; 2011.
30 Sundhedsstyrelsen. Opfølgningsprogrammer for kræftsygdomme [web site]. Oslo, Sundhedsstyrelsen; 2016 (https://sundhedsstyrelsen.dk/da/sundhed/folkesygdomme/kraeft/ opfoelgningsprogrammer, accessed 14 January 2017).
31 Jemal A et al. Global cancer statistics. CA: Cancer Journal for Clinicians, 2011;61(2):69–90. 32 Lewis RA et al. Follow-up of cancer in primary care versus secondary care: systematic review. British Journal of General Practice, 2009;59(564):e234–e247.
See part I of Community-level cancer care chapter