Community-level cancer care, part I
EU policy recommendations for quality improvement in cancer after-care at the community level
This is a part I of chapter Community-level cancer care (Cancon work package 7) of the Guide. See part II.
See the full Guide and other chapters as pdf's.
Cancon Guide is the main delivery of the joint action.
Contents of Community-level cancer care part I
Introduction
Methods
Results
Community-level cancer care part II
Explorative studies of after-care for cancer patients in five European countries
Discussion
Conclusions and recommendations
References
Introduction
What is cancer after-care? Cancer after-care is the period when care is provided to patients who are in remission and have completed the planned disease-specific oncological care. They are monitored for recurrence and late effects of treatment but can be generally classified as cured. The modalities of organization of this care differ largely across countries and may be through arrangements in hospitals and their outpatient departments, through community care providers or in a combination of the two.
One of the important positive effects and challenges associated with recent advances in cancer treatment has been the constant rise in the number of cancer survivors. These might be patients who are in long remission periods as well as patients who are receiving life-prolonging treatments. Increasing incidence and prevalence of cancers, through the ageing population and the increase in some risk factors (e.g. obesity, physical inactivity) is leading to an increasing demand for oncological care closer to home. Consequently, the importance of coordinating processes and patient pathways in cancer care across levels of care has been increasing. Grunfeld and Earle stressed the importance of “transitions”: from focus on diagnosis and treatment to long-term follow-up care, management of late effects, rehabilitation and health promotion (1). Throughout these transitions, collaboration between oncologists and GPs is crucial. This collaboration can be challenged by variable interest, poor communication with GPs and patient preferences for followup (2). The last issue also relates to differences in health systems and organizational preferences arising from a specific health care system (e.g. strong stress on hospital and specialist care, free access to specialist, low profile for PCPs).
After-care was traditionally in the hands of cancer centres and hospitals treating cancers. Increasing incidences, ageing of populations, increased complexity of the initial phases of cancer care and changing patterns of care at the community level now pose a challenge and the need to place an important share of cancer after-care in the hands of PCPs. One of the challenges arises from the costs of oncological care within cancer centres or specialized hospital departments. In addition, intensification of oncological care, particularly at the level of medical/clinical oncology, has meant focusing time and efforts on patients receiving treatment, to the detriment of the after-care phase.
A further consequence is that after-care is less well defined and, most importantly, less structured. Cancer patients also face social challenges – loss of work capacity, rehabilitation, disability and/or mental problems – which all require a comprehensive psychosocial approach close to a patient’s living and working environment. In view of these challenges, structured development of (new) pathways should ideally encompass the transfer of cancer after-care into community care settings along with adequate training of all health and social care professionals involved in the process.
Proper reallocation of tasks is necessary in order to secure an adequate quality of care, its continuity and a seamless process, as well as for maintaining a high level of patient trust and cooperation. In addition, most cancer patients are older and very often have chronic diseases before their cancer diagnosis, particularly cardiovascular diseases, diabetes or a neurological problem. Cancer survivors have different types of need that may be insufficiently met by general practice (3).
Two further terms need explanation and clarification with respect to the context in which they are used throughout this chapter – primary care and community care. It was decided to use the term community care as it encompasses the wider range of services necessary for any patient with a chronic condition. These services go beyond the immediate care delivered within health care and are specific in cancer patients because of the range of outcomes, including long remissions and cure. Community care also needs to address social, economic, employment, financial and spiritual needs of patients, which arise either from the longitudinal nature of the disease or from the consequences of the treatment and their impact of the patient’s daily life, economic, employment, social life and other activities. Care of patients at the community care level will be explored as a
challenge in changing health systems where rational approaches should ideally meet with patient preferences.
This chapter focuses on the organization of after-care and supportive care for patients, predominantly outside of specialized oncological care. The latter normally encompasses the specialized and focused curative treatment that is prescribed based on a baseline assessment of the patient’s disease. Once in remission and classified as without active disease, patients mostly return to their lives before cancer. Nevertheless, this phase and period pose different challenges, both for the patient and for health care services at the community care level. Chapter 7 will focus on patient experiences and will deal with the first group of challenges through an analysis of the
current state in this field as well and by proposing a survivorship care plan. Here we explore the organizational and process aspects of the organization of cancer after-care for those patients who require further interactions with community and social care because of their cancer treatment.
The chapter does not generally include patients requiring palliative and end-of-life care apart from the case study of Norway, where care for this group of patients is inseparably connected to the organization of after-care. The organizational framework provided by the health system is also one of the important levers to secure equitable access to necessary services, attempting to avoid any increase in differences among patients based on social, geographical, gender or other characteristics. The ambition here is to draw attention to this specific segment of cancer care in order to:
- define after-care for cancer patients better, including the different services (as required by patients and their needs) and the organization of community care for these patients, their spouses or significant others, carers and families;
- outline how after-care is organized in certain European countries and highlight positive experiences; and
- argue for the need to structure and carefully plan the entire span of the cancer patient pathway including after-care, with full support in terms of adequate resources.
The chapter presents an inventory of what information on after-care is available for GPs as their role expands and an overview of national and regional practices on how the phase of cancer after-care is organized in Bulgaria, Denmark, the Netherlands, Norway and Slovenia. Four of these countries have a GP gate-keeping system and a strong role of community care, including in the cancer after-care process. Only in Bulgaria does after-care remain the task of cancer hospitals.
Based on these findings, we will draw tentative conclusions, which will translate into draft policy recommendations for the future development of patient pathways for cancer after-care and its management in community care settings.
TopMethods
This chapter describes the analyses done within CanCon and incorporating findings of previous, current and ongoing research. Five European countries – Bulgaria, Denmark, the Netherlands, Norway, and Slovenia – were actively involved in its development by virtue of their participation in the project. The countries presented in this chapter were included given the interest of their respective ministries of health in participating in the project. This is, therefore, self-inclusion and not a systematic and structured involvement of specific countries. Following the self-inclusion, the methodologies used in the different countries in order to substantiate some specificities of
their after-care process, the relationship between levels of care and the relationship between central and regional activities vary greatly. Consequently, there was no overall harmonization of methodologies.
A short description of the methods is provided here, while a detailed description can be found online (supplemental information provided at www.cancercontrol.eu). Given the diversity of country cases and the respective foci of their research, a series of methods were used for data collection:
Survey of experts and country informants
A survey of experts and country informant was carried out with the collaboration of national contact points: project partners, ministries of health, cancer centres and public health institutes and similar services. Experts from all EU Member States, Norway, Switzerland, Iceland and Turkey participated in identifying guidelines as well as providing national or regional guidelines that included relevant information for GPs.2 Experts from 12 countries (Czechia, Estonia, Greece, Iceland, Latvia, Luxemburg, Malta, Slovenia, Slovakia, Sweden and Turkey) indicated that there were no tumour-specific guidelines containing information on after-care relevant to GPs.
The remaining experts indicated that there was at least one tumour-specific guideline. Despite multiple efforts, eight experts from seven different countries, who had indicated that their country had tumourspecific guidelines, did not provide any guideline. In total, 77 guidelines were received and 47 were deemed relevant. In databases and on web sites, 48 additional relevant guidelines were found.
Literature review of existing guidelines on after-care for breast, colorectal, lung, melanoma and prostate cancer survivors focused on the relevance of guidelines for GPs. International guidelines were collected via searches on the Internet and in literature to create a more complete overview of guidelines. Databases Embase and Medline, the National Guideline Clearinghouse (4) and the Guidelines International Network (5) were searched using the terms “guideline”, “breast cancer”, “colorectal cancer”, “colon cancer”, “rectum cancer”, “melanoma”, “lung cancer”, “prostate cancer”. In addition, cancer agency web sites were accessed for relevant tumour-specific guidelines.
A category and topic list per tumour type was composed after assessing all guidance on aftercare. The objective was to establish uniform categories and topics for the various tumour types. Several categories for the purpose of this study were defined: recurrence detection, long-term effects and recurrence prevention. The category and topic list can be found online (supplemental information provided at www.cancercontrol.eu). An important element was awareness, which means awareness of patients to potential recurrence, monitoring of disease development and of signs and symptoms of the disease. Guidance considered relevant was summarized into topics independently by two researchers. If guidance did not fit into the created topics or if topics became
too broad, a new topic was created by discussion. Disagreements arising from decisions on scoring were resolved by discussion with a third researcher.
Exploration of cancer after-care organization and services in five European countries
In Bulgaria, the National Centre for Public Health Analyses carried out a set of structured interviews on perceptions of after-care services, in particular from the point view of the type of provider. The questionnaire was based on the one developed in the first phase of the Slovene study and was focusing on the description of the provision of cancer after-care in Bulgaria, with the volumes of care and staffing in each cancer centre; no information was collected on the actual contents of care.
For Denmark, a reform of after-care is in progress. Plans on follow-up care for each main type of cancer are under preparation. These plans should address several topics regarding cancer aftercare. For the Netherlands, NIVEL carried out a series of health services research studies (see the country study report below) and measured the impact on after-care on the volume of care in a GP practice (i.e. the perspective of the service delivery). They specifically analysed these impacts from the provider perspective, measuring the increased workload, effect on carers and on other personnel delivering community care.
The Norwegian case constitutes a health services intervention study where the activities of an ongoing transformation (the transfer of certain specialist services including palliative care to the community level) are described.
The Slovene study combined a quantitative cross-sectional survey of a stratified random sample of 250 GPs practising in Slovenia, with semi-structured interviews conducted on a purposive sample of six physicians from the Upper Carniola region. GPs were interviewed about some characteristics of cancer patients on their lists and after-care delivered to these patients. One focus of this study was the exploration of links between different services in community care and social services.
Finally, good practice recommendations on after-care in GP practices were developed and tested during the study. The selection of countries included was not intended to be representative of all EU Member States
nor exhaustive. It has to be kept in mind that the exploration was not primarily research oriented, but rather seeking for good practices in the countries that participated in the CanCon project.
Results
After-care for cancer survivors: recommendations for GPs in cancer guideline
Given the growing number of cancer survivors worldwide, there are increasing calls for greater involvement of PCPs in after-care. Currently, there is only little structured information for GPs on the best way to provide after-care. Guidelines are an important source of information on after-care for GPs. Consequently, an investigation examined what information on after-care was available and (potentially) relevant for GPs in national and regional guidelines from European countries and nonEuropean western countries (Box 6.1). An inventory of tumour-specific guidelines on the five most common tumour types (breast cancer, colorectal cancer, lung cancer, melanoma and prostate cancer) was completed because it seemed likely that GPs see those survivors most frequently, and this would create an overview of all presented guidance and advice. Other tumour-specific
guidelines were reviewed to uncover additional information.
Many guidelines did not specify their target audience and so it was necessary to distinguish whether guidance was relevant for GPs. We chose to use the Dutch GP as a reference for what guidance was relevant; diagnostic tests and actions that Dutch GPs could perform were identified as potentially relevant. This included all actions that could be performed within the general practice, as well as tests that could be requested from a laboratory (blood tests) and routine screening tests where results are routinely sent to the GP (e.g. mammography). Expensive invasive diagnostic tests that hospitals normally provide (e.g. magnetic resonance imaging) were identified as irrelevant for GPs. The same applies for treatment of recurrence normally provided in secondary care.
In total, information on 95 guidelines (47 obtained via experts and 48 identified via literature and Internet searches) originating from 36 countries was extracted and summarized into topics (Table 6.1) (supplemental information provided at www.cancercontrol.eu, including a list of included guidelines and the providing countries)
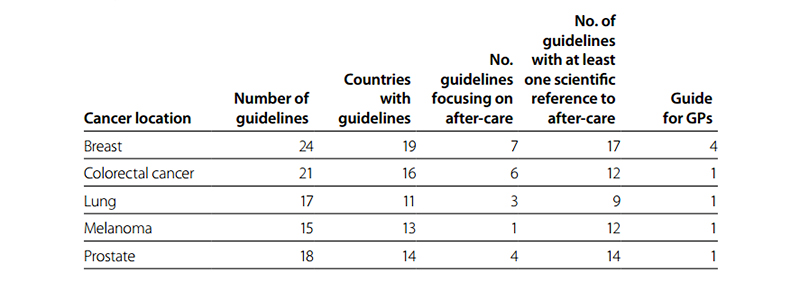
Table 6.1 Number of guidelines and their focus concerning after-care guidelines and providers
Breast cancer
All of the 24 guidelines related to breast cancer recommended performing physical diagnostic tests (history and physical examination) and diagnostic imaging (mammography) but showed less consensus about the frequency. All guidelines agreed on an annual examination after five years, which reflects that most recurrences happen within five years of treatment (6). Five of the 20 recommended time intervals were evidence based. These recommendations showed nearly the same inequality in consecutive time intervals; there was only more agreement on the intervals in the first two years after diagnostic tests (every three to six months). More agreement was observed on the frequency of mammography. Nineteen guidelines recommend performing a mammogram annually (supplemental information provided at www.cancercontrol.eu). Most of the guidelines
recommend check-ups every three to six months in the first three years after treatment, followed mostly by six-monthly check-ups until after the fifth year.
The breakdown of the different tests and procedures related to breast cancer after-care is presented in Fig. 6.1, including the weight given to the different tests. Tables 6.1 and 6.2 present the frequency of the key elements of the guidelines per number of the guidelines in which they had been identified (see above). Laboratory diagnostic tests were considered as non-routine tests. Only seven guidelines provided recommendations on self-examination by the patient.
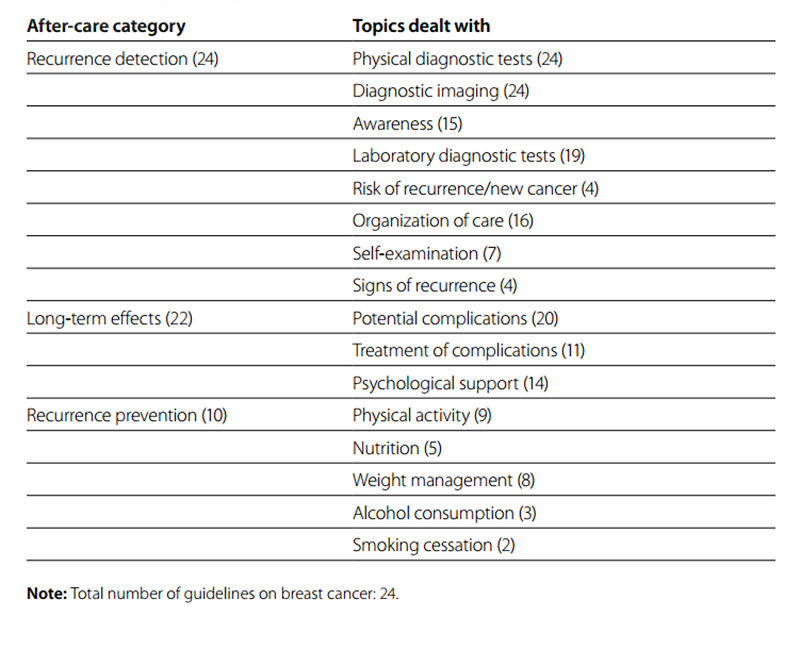
Table 6.2 Number of guidelines on breast cancer by after-care category and number of topics dealt with per category
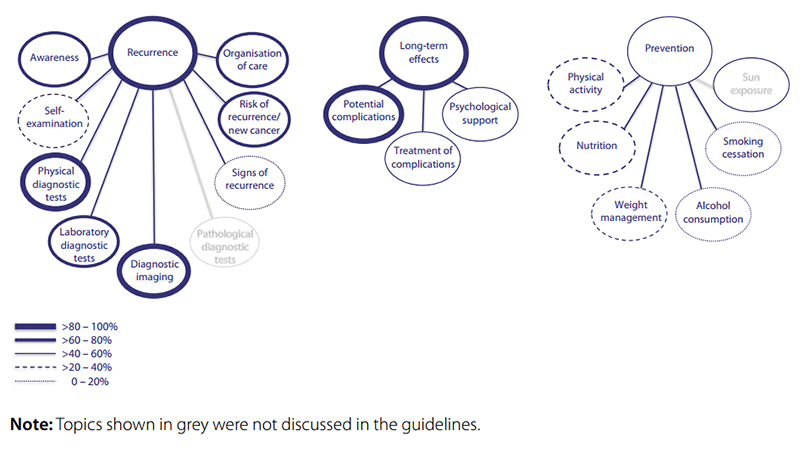 Fig. 6.1 Overview of categories and topics on after-care for breast cancer derived from 24 guidelines
Fig. 6.1 Overview of categories and topics on after-care for breast cancer derived from 24 guidelines
Colorectal cancer
Two guidelines were specific for colon cancer and two for rectal cancer. In total, information from 20 guidelines was used. Fig. 6.2 presents an overview of the potentially relevant categories and topics for GPs. All 20 guidelines provided information and recommendations on recurrence detection; seven topics in this category were identified. Laboratory diagnostic tests (carcinoembryonic antigen testing), physical diagnostic tests (history and physical examination), awareness and organization of care received most attention. One of the six after-care-specific guidelines discussed long-term effects such as depression/distress, fatigue and incontinence problems. Four guidelines discussed prevention of colorectal cancer recurrence and one guideline provided information on all five topics identified within prevention of colorectal cancer (Table 6.3).
Guidelines agreed on the usefulness of laboratory and physical diagnostic tests, but not on the time interval between consecutive tests (supplemental information provided at www.cancercontrol.eu). The majority of
recurrences occur within the first three years (7). There was high agreement to stop diagnostic tests after five years, except in one guideline. After five years, the risk of colorectal recurrence is very low; just less than 1% of all recurrences occur later than five years after surgery (8). Only three guidelines reported that the recommendation on physical diagnostic tests was evidence based.
For carcinoembryonic antigen testing, the same tendency towards less frequent tests after three years was observed, while all guidelines agreeing to stop after five years.
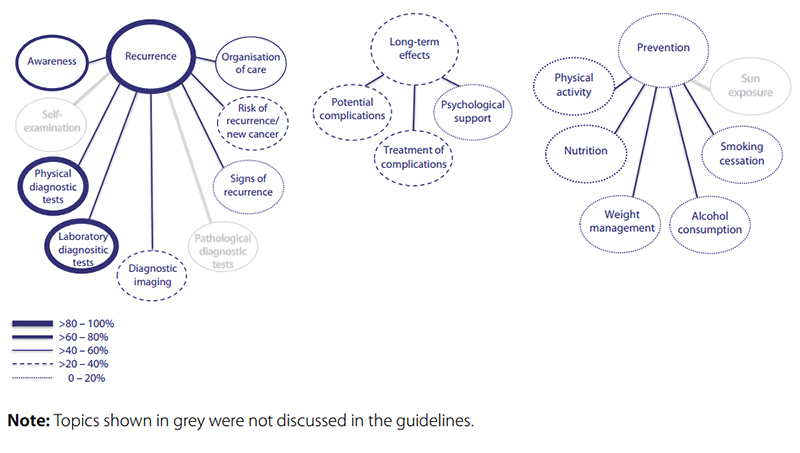
Fig. 6.2 Overview of categories and topics on after-care for colorectal cancer derived from 20 guidelines
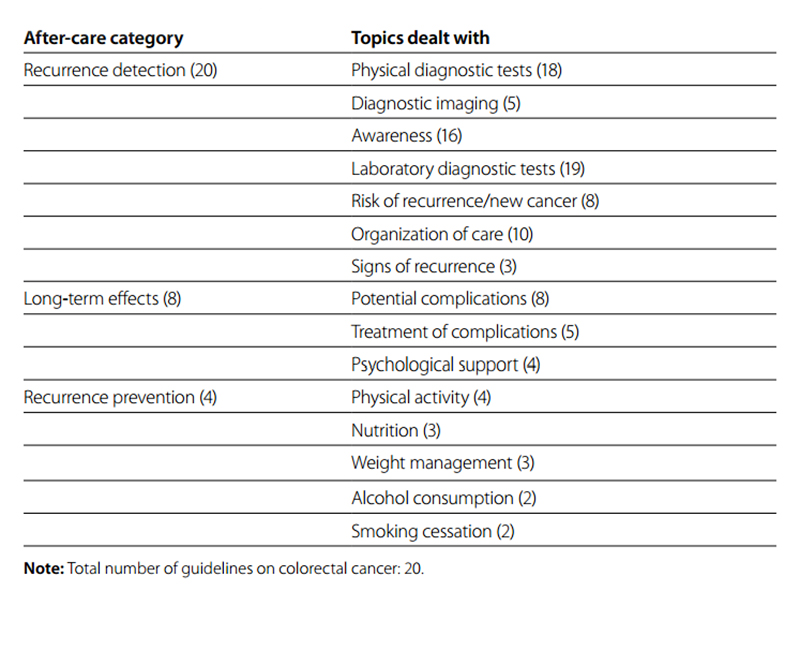
Table 6.3 Number of guidelines on colorectal cancer by after-care category and number of topics dealt with per category
Lung cancer
In total, 17 guidelines were used to create an overview. Eight out of 15 guidelines were on both small and non-small cell lung cancer, five were only on small cell lung cancer and two were on non-small lung cancer. Fig. 6.3 shows an overview of potentially relevant categories and topics for GPs on lung cancer, identified as present in the 15 guidelines with 15 topics. All guidelines discussed recurrence detection of lung cancer, giving information on seven topics among which physical diagnostic tests and awareness were most prominent (Table 6.4). Four guidelines discussed long-term effects of lung cancer (none of them after-care specific guidelines). The most reported potential complications were pain and loss of lung function, but only one guideline provided information on treatment of complications.
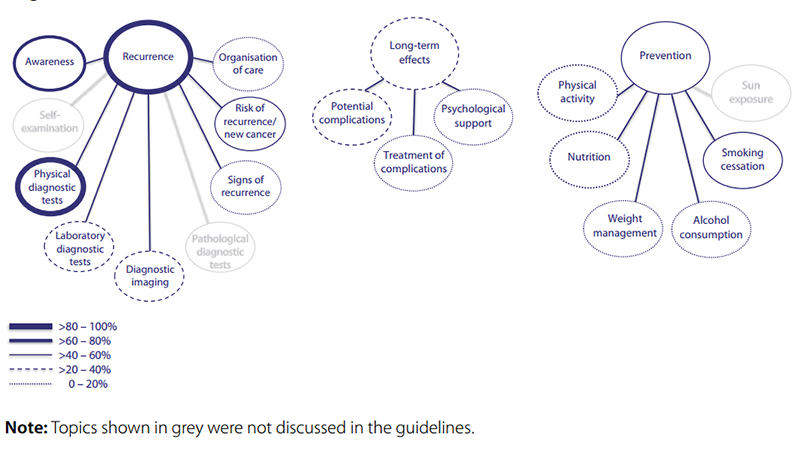
Fig. 6.3 Overview of categories and topics on after-care for lung cancer derived from 15 guidelines
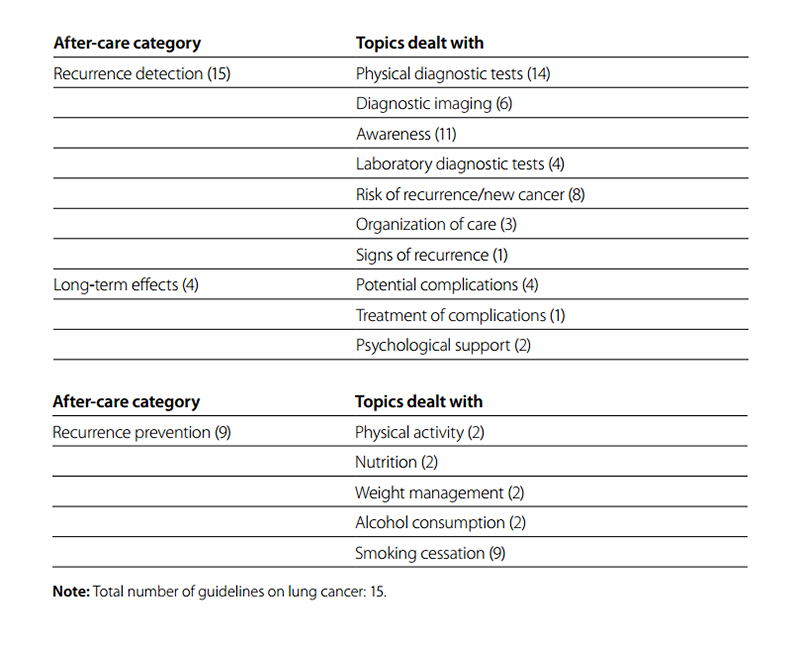 Table 6.4 Number of guidelines on lung cancer by after-care category and number of topics dealt with per category
Table 6.4 Number of guidelines on lung cancer by after-care category and number of topics dealt with per category
The recommended frequency was highest in the first year after treatment, corresponding to risk of lung cancer recurrence that peaks around nine months after treatment (9). Around 60–70% of the relapses occurred in the initial two to three years after treatment, reflected by the high frequency of follow-up during these years. In the fourth and fifth year, the risk of recurrence declines and recommended frequencies were once to twice a year.
After five years, most guidelines recommended annually diagnostic tests or to stop testing as the majority of relapses occurred in the first five years after treatment. The two guidelines that reported that the recommendation on physical diagnostic tests was evidence based did not agree on time intervals between diagnostic tests. One recommended physical examination every three to six months for three years and then annually, while the other recommended performing physical examination every three months in the first two years, every six months in year three to five and then annually. This means that a uniform position is needed from oncologists in order to inform the PCP.
TopMelanoma
All guidelines included recommendations on recurrence detection (Fig. 6.4). Of the eight topics identified, physical diagnostic tests, self-examination, risk of recurrence and laboratory diagnostic tests received most attention (Table 6.5). Melanoma stands out among the five cancer types in that self-examination is recommended as a method of recurrence detection. Eight guidelines discussed long-term effects of melanoma, focusing in particular on psychological support but also on potential treatment complications such as lymphoedema, fatigue, fear, depression/distress and thrombocytopenia. Only sun exposure was identified within the category recurrence prevention.
Six guidelines highlighted the avoidance of sunburn by reducing sun exposure and tanning use. All guidelines recommended physical diagnostic tests and 12 gave recommendations on time intervals. In eight of these, the recommended frequency depended on the stage of the primary melanoma, while four gave recommendations independent of the initial stage. As the risk of recurrence is related to the primary tumour thickness (10), the frequency of follow-up depends on the stage of the primary melanoma. There is no agreement on the time intervals between consecutive physical diagnostic tests. After 10 years, seven guidelines recommended stopping diagnostic tests, seven to continue testing and two to continue if diagnostic tests were clinically indicated. Four guidelines indicated that the recommended time intervals were evidence based.
There is somewhat more agreement on the time intervals if the initial stage of the melanoma was stage II or III. Time intervals are shorter compared with stage I because the risk of recurrence is higher (11). Most guidelines agreed on three to six month time intervals in the first three years after treatment. This is in accordance with the risk of recurrence, which is highest in the first two to three years after treatment (12).
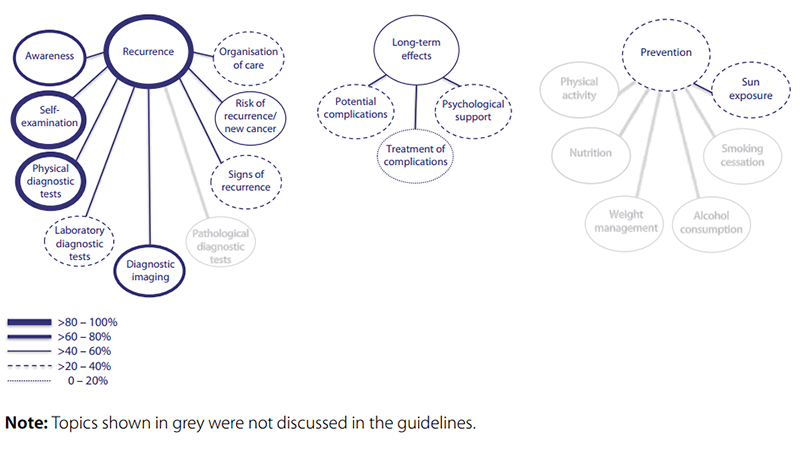
Fig. 6.4 Overview of categories and topics on after-care for melanoma derived from 15 guidelines
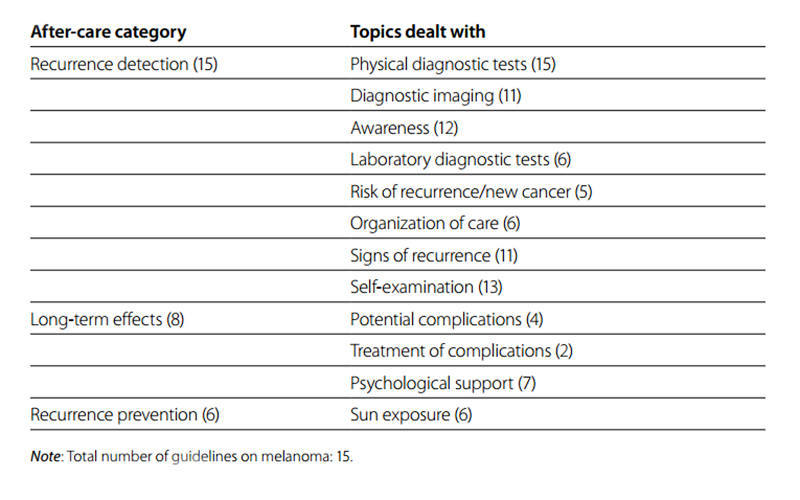
Table 6.5 Number of guidelines on melanoma by after-care category and number of topics dealt with per category
Prostate cancer
Recurrence detection was discussed in all guidelines (Fig. 6.5 and Table 6.6). Most attention was paid to the value of testing for PSA, followed by digital rectal examination and awareness; other topics were less well covered. More than half of the guidelines (11 of 18) discussed long-term effects of prostate cancer, where 11 guidelines mentioned at least one potential complication. Distinction was made between urinary, sexual, bowel and other complications; urinary incontinence and erectile dysfunction were most often mentioned.
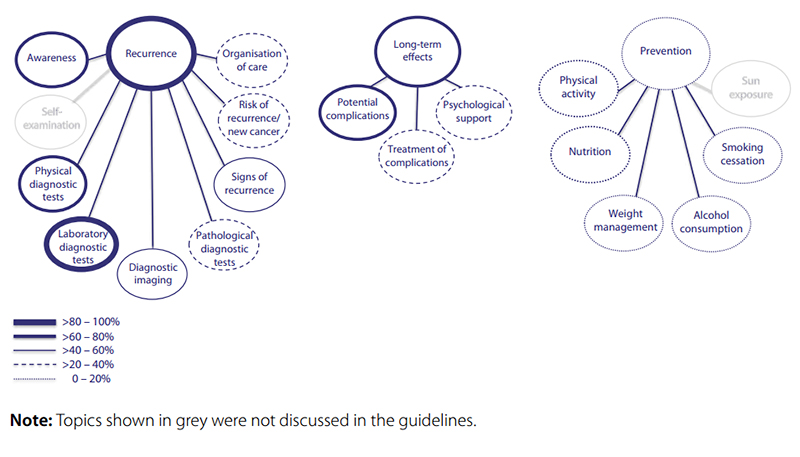
Fig. 6.5 Overview of categories and topics on after-care for prostate cancer derived from 18 guidelines
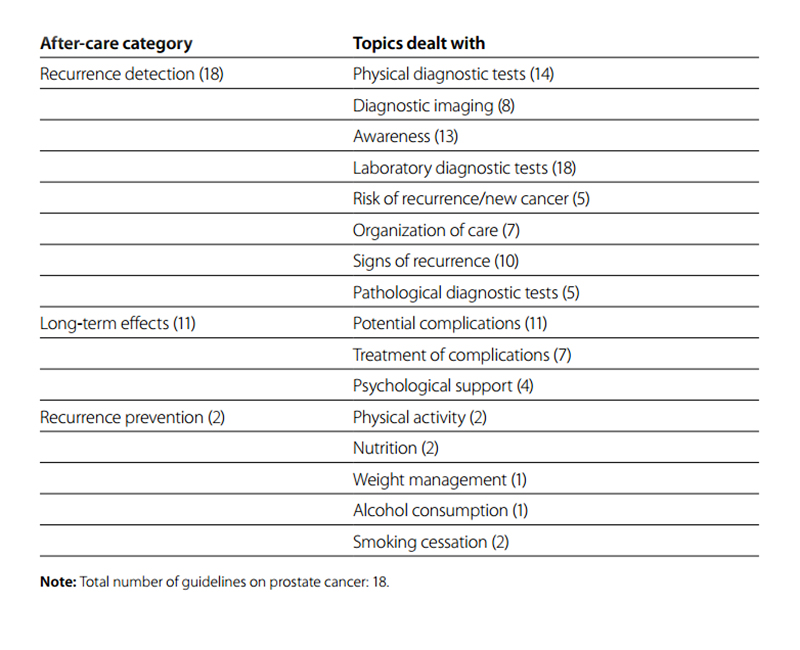
Table 6.6 Number of guidelines for prostrate cancer by after-care category and number of topics dealt with per category
There was not a high level of agreement across guidelines on the frequency of the two most important follow-up monitoring methods: digital rectal examination and PSA testing. The suggested frequency for digital rectal examination was either every six or every 12 months for the first three years after treatment. From the fourth year onwards, the predominant suggestion was for an annual check. There was no agreement on the frequency in the first years for PSA testing; for the second and third year guidelines leant towards six-monthly checks. In the fourth and fifth year, there was again no agreement, while after five years it was recommended as an annual check.
Summary
All guidelines noticed that the detection of recurrence is the most important part of after-care. Although it is known which diagnostic tests are best to detect recurrence, the best frequency to perform diagnostic tests to detect cancer recurrence is not known. Compared with recurrence detection, long-term effects of cancer got less attention in the guidelines. Most breast cancer guidelines (83.3%) and more than half of the prostate cancer guidelines (61.1%) reported potential complications of the specific cancer. Guidelines on colorectal cancer, lung cancer and melanoma provided only little information on long-term effects. Prevention of cancer recurrence received by far the least attention in the guidelines and it seems that prevention recommendations are not tumour or even cancer specific. This study does not provide the best practice on after-care for GPs,
but it shows the most complete practice and an overview of information on after-care (potentially) relevant for GPs.
See part II of Community-level cancer care chapter